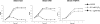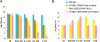Killing niche competitors by remote-control bacteriophage induction - PubMed (original) (raw)
Killing niche competitors by remote-control bacteriophage induction
Laura Selva et al. Proc Natl Acad Sci U S A. 2009.
Abstract
A surprising example of interspecies competition is the production by certain bacteria of hydrogen peroxide at concentrations that are lethal for others. A case in point is the displacement of Staphylococcus aureus by Streptococcus pneumoniae in the nasopharynx, which is of considerable clinical significance. How it is accomplished, however, has been a great mystery, because H(2)O(2) is a very well known disinfectant whose lethality is largely due to the production of hyperoxides through the abiological Fenton reaction. In this report, we have solved the mystery by showing that H(2)O(2) at the concentrations typically produced by pneumococci kills lysogenic but not nonlysogenic staphylococci by inducing the SOS response. The SOS response, a stress response to DNA damage, not only invokes DNA repair mechanisms but also induces resident prophages, and the resulting lysis is responsible for H(2)O(2) lethality. Because the vast majority of S. aureus strains are lysogenic, the production of H(2)O(2) is a very widely effective antistaphylococcal strategy. Pneumococci, however, which are also commonly lysogenic and undergo SOS induction in response to DNA-damaging agents such as mitomycin C, are not SOS-induced on exposure to H(2)O(2). This is apparently because they are resistant to the DNA-damaging effects of the Fenton reaction. The production of an SOS-inducing signal to activate prophages in neighboring organisms is thus a rather unique competitive strategy, which we suggest may be in widespread use for bacterial interference. However, this strategy has as a by-product the release of active phage, which can potentially spread mobile genetic elements carrying virulence genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
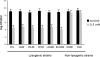
Fig. 1.
Killing of S. aureus by H2O2. Survival of lysogenic (COL, Mu50, FRI-S6, RF122, LUG855, and RN10359) or nonlysogenic (V329, RN450) S. aureus strains in media supplemented with hydrogen peroxide at a concentration of 0.5 mM, and unsupplemented medium (control). Values represent the average of 3 independent experiments. Variation was within ± 5% in all cases.
Fig. 2.
Lysis of S. pneumoniae induced by mitomycin C or H2O2. Cultures of lysogenic (623 and 949) or nonlysogenic (TIGR4) strains received mitomycin C (0.1 μg/mL) or H2O2 (0.5 mM) at time 0, and the OD of cultures was monitored at 600 nm.
Fig. 3.
Role of catalase in SOS induction by H2O2. (A) Survival of S. aureus strains in media supplemented with hydrogen peroxide at different concentrations. Values represent the average of 3 independent experiments. Variation was within ±5% in all cases. (B) Phage titer obtained from the lysogenic strains analyzed in A.
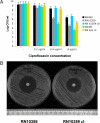
Fig. 4.
Killing of lysogenic S. aureus cells by antibiotics. (A) Survival S. aureus strains in media supplemented with different concentrations of the antibiotic ciprofloxacin. RN450, nonlysogenic; RN10359, RN450 lysogenic for phage 80α; RN10359 cI−, derivative of RN10359 carrying a non-SOS-inducible phage 80α; RN451, RN450 lysogenic for phage 11; RN451 cI−, derivative of RN451 carrying a non-SOS-inducible phage 11. Values represent the average of 3 independent experiments. Variation was within ± 5% in all cases. (B) Antibiogram showing the different susceptibility between strains RN10359 (lysogenic for phage 80α) and RN10359 cI− (carrying a non-SOS-inducible phage 80α) to enrofloxacin discs. Average zone of inhibition: RN10359, 36 mm; RN10359 cI−, 30 mm.
Similar articles
- Temperate phage-antibiotic synergy across antibiotic classes reveals new mechanism for preventing lysogeny.
Al-Anany AM, Fatima R, Nair G, Mayol JT, Hynes AP. Al-Anany AM, et al. mBio. 2024 Jun 12;15(6):e0050424. doi: 10.1128/mbio.00504-24. Epub 2024 May 17. mBio. 2024. PMID: 38757974 Free PMC article. - Antibiotics Stimulate Formation of Vesicles in Staphylococcus aureus in both Phage-Dependent and -Independent Fashions and via Different Routes.
Andreoni F, Toyofuku M, Menzi C, Kalawong R, Mairpady Shambat S, François P, Zinkernagel AS, Eberl L. Andreoni F, et al. Antimicrob Agents Chemother. 2019 Jan 29;63(2):e01439-18. doi: 10.1128/AAC.01439-18. Print 2019 Feb. Antimicrob Agents Chemother. 2019. PMID: 30509943 Free PMC article. - Influence of SOS-inducing agents on the expression of ArtAB toxin gene in Salmonella enterica and Salmonella bongori.
Miura S, Tamamura Y, Takayasu M, Sasaki M, Nishimura N, Tokugawa K, Suwa I, Murata R, Akiba M, Kusumoto M, Uchida I. Miura S, et al. Microbiology (Reading). 2020 Aug;166(8):785-793. doi: 10.1099/mic.0.000939. Epub 2020 Jun 24. Microbiology (Reading). 2020. PMID: 32579098 - Role of Staphylococcus aureus catalase in niche competition against Streptococcus pneumoniae.
Park B, Nizet V, Liu GY. Park B, et al. J Bacteriol. 2008 Apr;190(7):2275-8. doi: 10.1128/JB.00006-08. Epub 2008 Jan 25. J Bacteriol. 2008. PMID: 18223076 Free PMC article. - The SOS system.
d'Ari R. d'Ari R. Biochimie. 1985 Mar-Apr;67(3-4):343-7. doi: 10.1016/s0300-9084(85)80077-8. Biochimie. 1985. PMID: 2994755 Review.
Cited by
- Streptococcus pneumoniae Eradicates Preformed Staphylococcus aureus Biofilms through a Mechanism Requiring Physical Contact.
Khan F, Wu X, Matzkin GL, Khan MA, Sakai F, Vidal JE. Khan F, et al. Front Cell Infect Microbiol. 2016 Sep 27;6:104. doi: 10.3389/fcimb.2016.00104. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27730096 Free PMC article. - Composition and immunological significance of the upper respiratory tract microbiota.
Schenck LP, Surette MG, Bowdish DM. Schenck LP, et al. FEBS Lett. 2016 Nov;590(21):3705-3720. doi: 10.1002/1873-3468.12455. Epub 2016 Nov 1. FEBS Lett. 2016. PMID: 27730630 Free PMC article. Review. - Evidence of in vivo prophage induction during Clostridium difficile infection.
Meessen-Pinard M, Sekulovic O, Fortier LC. Meessen-Pinard M, et al. Appl Environ Microbiol. 2012 Nov;78(21):7662-70. doi: 10.1128/AEM.02275-12. Epub 2012 Aug 24. Appl Environ Microbiol. 2012. PMID: 22923402 Free PMC article. - Transcriptional profiling of Pseudomonas aeruginosa and Staphylococcus aureus during in vitro co-culture.
Tognon M, Köhler T, Luscher A, van Delden C. Tognon M, et al. BMC Genomics. 2019 Jan 10;20(1):30. doi: 10.1186/s12864-018-5398-y. BMC Genomics. 2019. PMID: 30630428 Free PMC article. - Acetic acid increases the phage-encoded enterotoxin A expression in Staphylococcus aureus.
Wallin-Carlquist N, Cao R, Márta D, da Silva AS, Schelin J, Rådström P. Wallin-Carlquist N, et al. BMC Microbiol. 2010 May 20;10:147. doi: 10.1186/1471-2180-10-147. BMC Microbiol. 2010. PMID: 20487538 Free PMC article.
References
- Bassler BL, Losick R. Bacterially speaking. Cell. 2006;125:237–246. - PubMed
- Regev-Yochay G, et al. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in Children. JAMA. 2004;292:716–720. - PubMed
- Bogaert D, et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871–1872. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources