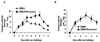Resveratrol enhances cell-mediated immune response to DMBA through TLR4 and prevents DMBA induced cutaneous carcinogenesis - PubMed (original) (raw)
Resveratrol enhances cell-mediated immune response to DMBA through TLR4 and prevents DMBA induced cutaneous carcinogenesis
Nabiha Yusuf et al. Mol Carcinog. 2009 Aug.
Abstract
Toll-like receptors (TLRs) activate signals that are critically involved in innate immune responses and that contribute to the initiation of adaptive immune responses. Resveratrol (trans-3,5,4-trihydroxystilbene), a polyphenol found in red grapes and in several other plant sources, is an effective chemopreventive agent in cutaneous chemical carcinogenesis. In this study, we investigated whether TLR4 was required for the chemopreventive action of resveratrol in DMBA skin carcinogenesis. For this purpose, mice with normal and deficient TLR4 function were compared when pretreated with resveratrol and then subjected to a DMBA-induced skin carcinogenesis protocol. There were fewer tumors/group (P < 0.001) in resveratrol treated TLR4 competent C3H/HeN mice than in TLR4 deficient C3H/HeJ mice. In addition, the size of tumors in C3H/HeN mice was reduced in vivo and their survival in vitro was inhibited by resveratrol to a significantly greater extent than in C3H/HeJ mice. Resveratrol inhibited angiogenesis to a much greater extent in the TLR4 competent mice than in TLR4 deficient mice. IFN-gamma and IL-12 levels were also increased in TLR4 competent mice compared to TLR4 deficient mice, and TLR4 competent C3H/HeN mice exhibited a greater increase in the cell-mediated immune response to DMBA. The results of this study indicate that TLR4 is an important mediator of resveratrol chemoprevention in DMBA skin tumorigenesis.
Figures
Figure 1
Resveratrol suppresses DMBA induced tumor formation in a TLR4 dependent manner. TLR4 deficient C3H/HeJ mice and TLR normal C3H/HeN mice were subjected to a DMBA complete cutaneous carcinogenesis protocol (15 mice/panel) as described in the Methods section. The number of tumors per group was plotted as a function of the number of weeks on the test. There were significantly fewer tumors in C3H/HeN mice (**p<0.001) after treatment with resveratrol but there was no inhibition of tumor formation in C3H/HeJ mice (A&B).The percentage of mice with tumors was plotted as a function of the number of weeks on the test. There were significantly lower percentage of C3H/HeN mice (**p<0.001) with tumors after treatment with resveratrol compared to C3H/HeJ mice (C&D).
Figure 2
Resveratrol reduces the growth of DMBA-induced tumors in a TLR4 dependent fashion. (A) The tumor volume was measured at various times after the initiation of treatment in C3H/HeN mice, and was significantly less in the resveratrol group than in the vehicle treated panel (*p<0.05). (B) The tumor volume was measured at various times after the initiation of treatment in C3H/HeJ mice, and was not significantly different in the resveratrol group than in the vehicle treated panel. (C, D and E) Tumor cells were obtained from DMBA-induced tumors from C3H/HeN or C3H/HeJ mice and were subjected to an MTT assay after 1, 2 or 3 days of resveratrol treatment as described in Methods section. The proliferative potential of tumor cells is expressed in terms of absorbance of each sample. The effect of resveratrol on the proliferative capacity was higher in tumor cells obtained from C3H/HeN mice when compared with tumor cells derived from C3H/HeJ mice. Results are the mean ± SD of triplicate cultures and each experiment was repeated three times. The Graph is representative of one of them.
Figure 2
Resveratrol reduces the growth of DMBA-induced tumors in a TLR4 dependent fashion. (A) The tumor volume was measured at various times after the initiation of treatment in C3H/HeN mice, and was significantly less in the resveratrol group than in the vehicle treated panel (*p<0.05). (B) The tumor volume was measured at various times after the initiation of treatment in C3H/HeJ mice, and was not significantly different in the resveratrol group than in the vehicle treated panel. (C, D and E) Tumor cells were obtained from DMBA-induced tumors from C3H/HeN or C3H/HeJ mice and were subjected to an MTT assay after 1, 2 or 3 days of resveratrol treatment as described in Methods section. The proliferative potential of tumor cells is expressed in terms of absorbance of each sample. The effect of resveratrol on the proliferative capacity was higher in tumor cells obtained from C3H/HeN mice when compared with tumor cells derived from C3H/HeJ mice. Results are the mean ± SD of triplicate cultures and each experiment was repeated three times. The Graph is representative of one of them.
Figure 3
Resveratrol reduces CD31 expression in tumors from DMBA treated mice. Samples of tumors and normal skin from C3H HeN and C3H HeJ mice were examined for CD31 fluorescence staining. DMBA-induced tumors from C3H/HeJ mice had higher levels of CD31 positive staining than tumors induced in C3H HeN mice. Upon resveratrol treatment the CD31 expression in C3H HeN mice was greatly reduced while in C3H HeJ mice there was no effect of resveratrol on CD31 expression. (A) Representative photomicrographs are shown from experiments conducted in tumor/skin samples from at least five mice in each group. Identical patterns were observed in all of the tumors examined. CD31-positive staining is indicated by bright pink fluorescence (Bar = 100 µm). (B) Graph showing CD31 intensity from all the groups from five different samples. *P<0.05.
Figure 4
Resveratrol inhibition of angiogenesis in DMBA treated mice is dependent on TLR4. (A)VEGF was measured in tumor lysates from mice placed on a DMBA cutaneous carcinogenesis protocol for 25 weeks after resveratrol treatment. TLR4 deficient C3H/HeJ mice showed significantly higher levels of VEGF in their tumor lysates as compared to TLR4 normal C3H/HeN mice as evaluated by ELISA. Upon resveratrol treatment there was significant decrease in VEGF in C3H HeN mice than C3H HeJ mice indicating TLR4 is important in resveratrol mediated decrease of angiogenic factors like VEGF. The results are expressed as ng of VEGF per milligram protein. (B) C3H HeJ mice express higher levels of mRNA of VEGF in DMBA treated tumors compared to C3HHeN mice as evaluated by Real Time PCR. Upon resveratrol treatment there was no change in mRNA levels of C3H HeJ mice while the VEGF levels in C3H HeN mice had greatly reduced. RNA was isolated and mRNA expression of VEGF was determined using real-time PCR with SYBR Green as the fluorescent dye as described in Materials and Methods. The results are presented as the relative expression of the individual mRNA with normalization to GAPDH. (C) Resveratrol also effectively inhibited MMP-2 and -9 mRNA expressions in DMBA induced tumors in C3H/HeN mice but did not do so in C3H/HeJ mice. Densitometry of MMP-2 and -9 expressions in tumors can be seen as the values under each band. The values shown are arbitrary. In each case, the values for control group (DMBA treated C3H HeJ group) was taken as "1" and comparison was then made with densitometry values obtained with other groups. Each experiment was repeated three times. (*p<0.05). The figure is representative of one of them. (D) C3H HeJ mice express higher levels of mRNA of MMP-2 and MMP-9 in DMBA treated tumors compared to C3HHeN mice as evaluated by Real Time PCR. Upon resveratrol treatment there was no change in mRNA levels of C3H HeJ mice while the MMP levels in C3H HeN mice had greatly reduced. RNA was isolated and mRNA expression of MMP-2 and MMP-9 was determined using real-time PCR with SYBR Green as the fluorescent dye as described in Materials and Methods. The results are presented as the relative expression of the individual mRNA with normalization to GAPDH.
Figure 4
Resveratrol inhibition of angiogenesis in DMBA treated mice is dependent on TLR4. (A)VEGF was measured in tumor lysates from mice placed on a DMBA cutaneous carcinogenesis protocol for 25 weeks after resveratrol treatment. TLR4 deficient C3H/HeJ mice showed significantly higher levels of VEGF in their tumor lysates as compared to TLR4 normal C3H/HeN mice as evaluated by ELISA. Upon resveratrol treatment there was significant decrease in VEGF in C3H HeN mice than C3H HeJ mice indicating TLR4 is important in resveratrol mediated decrease of angiogenic factors like VEGF. The results are expressed as ng of VEGF per milligram protein. (B) C3H HeJ mice express higher levels of mRNA of VEGF in DMBA treated tumors compared to C3HHeN mice as evaluated by Real Time PCR. Upon resveratrol treatment there was no change in mRNA levels of C3H HeJ mice while the VEGF levels in C3H HeN mice had greatly reduced. RNA was isolated and mRNA expression of VEGF was determined using real-time PCR with SYBR Green as the fluorescent dye as described in Materials and Methods. The results are presented as the relative expression of the individual mRNA with normalization to GAPDH. (C) Resveratrol also effectively inhibited MMP-2 and -9 mRNA expressions in DMBA induced tumors in C3H/HeN mice but did not do so in C3H/HeJ mice. Densitometry of MMP-2 and -9 expressions in tumors can be seen as the values under each band. The values shown are arbitrary. In each case, the values for control group (DMBA treated C3H HeJ group) was taken as "1" and comparison was then made with densitometry values obtained with other groups. Each experiment was repeated three times. (*p<0.05). The figure is representative of one of them. (D) C3H HeJ mice express higher levels of mRNA of MMP-2 and MMP-9 in DMBA treated tumors compared to C3HHeN mice as evaluated by Real Time PCR. Upon resveratrol treatment there was no change in mRNA levels of C3H HeJ mice while the MMP levels in C3H HeN mice had greatly reduced. RNA was isolated and mRNA expression of MMP-2 and MMP-9 was determined using real-time PCR with SYBR Green as the fluorescent dye as described in Materials and Methods. The results are presented as the relative expression of the individual mRNA with normalization to GAPDH.
Figure 5
Effect of resveratrol on DMBA contact hypersensitivity (CHS) in TLR4 deficient C3H/HeJ and C3H/HeN mice. (A) C3H/HeN mice were pretreated with resveratrol and sensitized with DMBA and ear challenged five days later as described in the Methods section. The CHS response was significantly higher in resveratrol pre-treated C3H/HeN mice than in vehicle pretreated C3H/HeN mice (*p<0.05). (B) C3H/HeJ mice were pretreated with resveratrol and sensitized with DMBA and ear challenged five days later as described in the Methods section. The CHS response was not significantly different in resveratrol pre-treated C3H/HeJ mice than in vehicle pretreated C3H/HeJ mice (*_p_>0.05). Results are the mean ± SD with 3 mice per group and each experiment was repeated twice with similar results. The graph is representative of one of them (*p<0.05).
Figure 6
IFN-γ and IL-12 production in skin lysates from C3H/HeN and C3H/HeJ mice after having been subjected to a DMBA cutaneous carcinogenesis protocol for 25 weeks with or without resveratrol treatment. (A) Skin lysates from resveratrol treated C3H/HeN mice showed higher levels of IFN-γ than vehicle pretreated C3H/HeN, whereas IFN-γ levels in skin lysates from resveratrol pretreated C3H/HeJ were not increased (*p<0.05). (B) Skin lysates from resveratrol treated C3H/HeN mice showed higher levels of IL-12 than vehicle pretreated C3H/HeN, whereas IL-12 levels in skin lysates from resveratrol pretreated C3H/HeJ were decreased compared to vehicle pretreated C3H/HeJ mice (*p<0.05). Results are the mean ± SD of panels containing 4 mice per group. Each experiment was repeated twice with similar results.
Similar articles
- Protective role of Toll-like receptor 4 during the initiation stage of cutaneous chemical carcinogenesis.
Yusuf N, Nasti TH, Long JA, Naseemuddin M, Lucas AP, Xu H, Elmets CA. Yusuf N, et al. Cancer Res. 2008 Jan 15;68(2):615-22. doi: 10.1158/0008-5472.CAN-07-5219. Cancer Res. 2008. PMID: 18199559 Free PMC article. - Cell mediated immune responses through TLR4 prevents DMBA-induced mammary carcinogenesis in mice.
Naseemuddin M, Iqbal A, Nasti TH, Ghandhi JL, Kapadia AD, Yusuf N. Naseemuddin M, et al. Int J Cancer. 2012 Feb 15;130(4):765-74. doi: 10.1002/ijc.26100. Epub 2011 Jun 18. Int J Cancer. 2012. PMID: 21455984 Free PMC article. - Acquired and innate immunity to polyaromatic hydrocarbons.
Yusuf N, Timares L, Seibert MD, Xu H, Elmets CA. Yusuf N, et al. Toxicol Appl Pharmacol. 2007 Nov 1;224(3):308-12. doi: 10.1016/j.taap.2006.12.009. Epub 2006 Dec 19. Toxicol Appl Pharmacol. 2007. PMID: 17258781 Free PMC article. Review. - The multiple mechanisms of cell death triggered by resveratrol in lymphoma and leukemia.
Frazzi R, Tigano M. Frazzi R, et al. Int J Mol Sci. 2014 Mar 20;15(3):4977-93. doi: 10.3390/ijms15034977. Int J Mol Sci. 2014. PMID: 24658441 Free PMC article. Review.
Cited by
- A comprehensive review on natural phenolic compounds as alternatives to in-feed antibiotics.
Gao J, Yang Z, Zhao C, Tang X, Jiang Q, Yin Y. Gao J, et al. Sci China Life Sci. 2023 Jul;66(7):1518-1534. doi: 10.1007/s11427-022-2246-4. Epub 2022 Dec 28. Sci China Life Sci. 2023. PMID: 36586071 Review. - Resveratrol: Twenty Years of Growth, Development and Controversy.
Pezzuto JM. Pezzuto JM. Biomol Ther (Seoul). 2019 Jan 1;27(1):1-14. doi: 10.4062/biomolther.2018.176. Biomol Ther (Seoul). 2019. PMID: 30332889 Free PMC article. Review. - Modulation of TLR/NF-κB/NLRP Signaling by Bioactive Phytocompounds: A Promising Strategy to Augment Cancer Chemotherapy and Immunotherapy.
Fakhri S, Moradi SZ, Yarmohammadi A, Narimani F, Wallace CE, Bishayee A. Fakhri S, et al. Front Oncol. 2022 Mar 1;12:834072. doi: 10.3389/fonc.2022.834072. eCollection 2022. Front Oncol. 2022. PMID: 35299751 Free PMC article. - Resveratrol: Biological and pharmaceutical properties as anticancer molecule.
Hsieh TC, Wu JM. Hsieh TC, et al. Biofactors. 2010 Sep-Oct;36(5):360-9. doi: 10.1002/biof.105. Biofactors. 2010. PMID: 20623546 Free PMC article. Review. - Polyphenols as Immunomodulatory Compounds in the Tumor Microenvironment: Friends or Foes?
Focaccetti C, Izzi V, Benvenuto M, Fazi S, Ciuffa S, Giganti MG, Potenza V, Manzari V, Modesti A, Bei R. Focaccetti C, et al. Int J Mol Sci. 2019 Apr 6;20(7):1714. doi: 10.3390/ijms20071714. Int J Mol Sci. 2019. PMID: 30959898 Free PMC article. Review.
References
- Takeda K, Kaisho T, Akira S. Annu Rev Immunol. 2003;21:335–376. - PubMed
- Byrd-Liefer CA, Block EF, Takeda K, Akira S, Ding A. Eur J Immunol. 2001;31:2448–2457. - PubMed
- Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., III The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. - PubMed
- Ohashi K, Burkart V, Flohé S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AR050948/AR/NIAMS NIH HHS/United States
- P30 AR050948-05/AR/NIAMS NIH HHS/United States
- P30 CA013148/CA/NCI NIH HHS/United States
- P30CA13148/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical