The insulin/Akt signaling pathway is targeted by intracellular beta-amyloid - PubMed (original) (raw)
The insulin/Akt signaling pathway is targeted by intracellular beta-amyloid
Han-Kyu Lee et al. Mol Biol Cell. 2009 Mar.
Abstract
Intraneuronal beta-amyloid (Abeta(i)) accumulates early in Alzheimer's disease (AD) and inclusion body myositis. Several organelles, receptor molecules, homeostatic processes, and signal transduction components have been identified as sensitive to Abeta. Although prior studies implicate the insulin-PI3K-Akt signaling cascade, a specific step within this or any essential metabolic or survival pathway has not emerged as a molecular target. We tested the effect of Abeta42 on each component of this cascade. In AD brain, the association between PDK and Akt, phospho-Akt levels and its activity were all decreased relative to control. In cell culture, Abeta(i) expression inhibited both insulin-induced Akt phosphorylation and activity. In vitro experiments identified that beta-amyloid (Abeta), especially oligomer preparations, specifically interrupted the PDK-dependent activation of Akt. Abeta(i) also blocked the association between PDK and Akt in cell-based and in vitro experiments. Importantly, Abeta did not interrupt Akt or PI3K activities (once stimulated) nor did it affect more proximal signal events. These results offer a novel therapeutic strategy to neutralize Abeta-induced energy failure and neuronal death.
Figures
Figure 1.
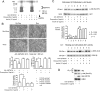
Akt activity and association with PDK: reductions in AD brain. Control brain: C1–3; AD brain: A1–3. (A) Top panels, p-Akt; levels of activated Akt (pThr308-Akt) are decreased in AD brain (cases A1–A3). Middle panels, Akt activity; a synthetic GSK-3β/substrate was less phosphorylated by IP Akt from AD brain samples (100 μg protein). Bottom graphs, quantitations of p-Akt and activity: Both p-Akt level and activity divided by total GSK were normalized over two AD brain sets (A1–A3 and A4–A6) using total Akt input. **p < 0.01 versus control; n = 6 (two-tailed Student's t test). (B) Akt and PDK interactions. Aβ coIPs with PDK and Akt1 in AD brain: top panel, IP PDK fractionated to detect association with Akt1 or pS473-Akt. Reverse IP shown below. Bottom panel, IPs of Akt, PDK and Aβ were probed with MAb 6E10 versus Aβ1-42. Synthetic Aβ peptide (50 ng) run as standard. (C) Control IPs of PKA with PDK and Akt1, and Aβ with PKA and NSE in AD brain are negative. IPs of PDK and Akt1 were probed with PKA Ab, and IPs of PKA and NSE were probed with MAb 6E10 versus Aβ1-42. Hsp27, a known Akt binding protein, is unchanged in AD samples. Synthetic Aβ peptide (50 ng) run as standard. NSE levels in whole tissue lysates demonstrate equal starting material.
Figure 2.
Intracellular Aβ (Aβi) in C2C12 myotubes: toxicity and inhibition of Akt activation. (A) Aβ1-42 expression. Myotube cultures were infected with Adenovirus Aβ42/TetOn and induced by doxycycline (Dox; 1 μg/ml). Cultures were treated with insulin (0.1 μg/ml) for 15 min before harvest. Monomers and oligomers are detected only in Dox-induced cultures. (B) Viability. Aβi expression decreases myotube length and caliber and increases pyknotic nuclei. Five random 20× fields were manually counted in each sample. + p < 0.05 versus lane 1; *p < 0.05 versus lane 3; **p < 0.01 versus lane 3; ***p < 0.001 versus lane 3; n = 4 (two-tailed Student's t test). (C) Aβ prevents Akt activation. C2C12 myotube cultures infected with AdvAβ42/TetOn were stimulated for 20 min with insulin, harvested, and used to IP Akt1. Phospho-Akt (Ser473) levels are reduced (top panels) in Aβ-expressing/insulin-stimulated cells (lane 6). Without insulin, a smaller but still significant decrease in Akt phosphorylation occurs (lane2). Quantitation of pSer473 and pThr308 Akt Western signals is shown below. *p < 0.05; **p < 0.01; n = 4 (two-tailed Student's t test). Lower panels, cell-based Akt kinase activity assay. Immunoblot of p-GSK-3α/β shows significantly inhibited phosphorylation in cultures treated with insulin. (D) Autophosphorylation of the insulin receptor is unchanged. Anti-phosphotyrosine IP from 100 μg myotube extracts infected with Adv Aβ/TetOn. Phosphorylation of IRβ was unaffected in Aβ-expressing cultures.
Figure 3.
In vitro–coupled assays and PDK-dependent phosphorylation and activation of Akt1. Effects of Aβ monomer, ADDL and fibril preparations. (A) Akt activity in three cell types. Three cell extracts (SH-SY5Y, HEK 293, and C2C12) were used to IP Akt and PDK for activity assays in the presence of synthetic, predominantly monomeric Aβ. In all cell types, PDK-dependent Akt activation was reversed by peptide. (B) Aβ specificity and dose dependence of inhibition in neuronal cells. IP PDK and IP Akt1 from SH-SY5Y cell extracts were mixed, and Akt activation was examined in vitro. pSer473 and pThr308 levels and activity to phosphorylate GSK-3α/β are affected by forward sequence (F) Aβ1-42 peptide (left panel), beginning at ∼1 μM (right panel). R, reverse Aβ (Aβ42-1). (C) Amyloid conformers have relative effects on PDK-dependent activation of Akt. Top panel, IP PDK and IP Akt1 from SH-SY5Y cells. Total amounts of Aβ in ADDL preparations are indicated. [ADDL] was estimated from the ratio of 12-mer to monomer in Supplemental Figure S2B. ADDLs that were aged 48 h more significantly prevented the activation of Akt. Bottom panel and Supplemental Figure S2C, fibril and ADDL-containing preparations, tested side-by-side.
Figure 4.
Specific properties of Aβ-directed inhibition of PDK activity. (A) Quantification of PDK-dependent Akt1 phosphorylation and activation. Dose-dependent Aβ inhibition of Akt phosphorylation (left graph) and Akt activation (middle graph) expressed as percent of control reactions without added Aβ42. *p < 0.05; **p < 0.01; ***p < 0.001; two-tailed Student's t test vs. reverse peptide (R) results. ADDL preparations aged 48 h (2.3 and 4.5 μM; estimated oligomers) were significantly more active against PDK activity than monomers (right graph). Data represent the analysis of results in Figure 3 as well as experiments not shown (n = 6). (B) Role of second messenger, PIP3. IP PDK from insulin-treated (INS) C2C12 myotubes was added to IP Akt from SH-SY5Y cells. Akt1-beads were preincubated with PP2A. The mixing of PDK and Akt1 led to submaximal increases in p-Akt (Ser473) and Akt activity (lane 3), an effect further enhanced by the addition of PIP3 (lane 4). Inclusion of ADDL nearly eliminates PDK alone or PDK plus added PIP3-induced activations (lanes 5 and 6). (C) PIP3 tethered to Akt and PDK IPs is sufficient for activation. Aβ is not acting like a phosphatase. IP Akt1 and IP PDK preincubated in phosphatase buffer alone (1× PB) or with added PTEN (10 μg/ml) or Aβ. PDK addition increased Akt activity as expected in control coupled kinase reactions (lane 3). Combining PTEN-treated PDK and Akt1 beads eliminated stimulation of Akt activity (lane 6 vs. 4). Preincubation of the two IP kinases with Aβ, followed by washout, produced no inhibitory effect (lane 8).
Figure 5.
Pulldown type PDK-Akt1 interaction assays. (A) Cell based. IP Akt1 from Aβ-expressing myotubes shows a decrease in coimmunoprecipitated PDK compared with control. Total Akt1 and PDK levels remain constant irrespective of Aβ (input). (B) Cell-free conditions. Akt-enriched cell extracts are prepared from myotube cultures infected with Adv WT-Akt. Extracts from unstimulated, control cultures are relatively abundant in PDK. Western blots of whole cell lysates (WCL) confirm expected levels of Akt and PDK (right panel). Cell extracts, 100 μg, each from Akt-enriched and control cultures, were mixed and incubated. Pulldown of PDK1 with Akt1 was significantly increased in the mixture of extracts (lane 3) but was abrogated by Aβ42 (lane 4). (C) In vitro interaction assay using PDK-modified cell extracts. Akt-enriched: PDK-depleted extracts are prepared by removing PDK through IP from Adv WT-Akt–expressing cell extracts. Control: Akt depleted lysates are prepared by removing Akt1 through IP from control cell extracts. Their respective levels are shown in lanes 3 and 4. These Akt1- and relative PDK-enriched fractions were mixed as before. The reverse IP of PDK pulled down less Akt1 and IP of Akt1 (as above) pulled down less PDK, in the presence of Aβ42.
Figure 6.
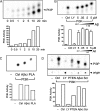
Negative effects to reverse established Akt activation by Aβi; extracellular applications. (A) Preactivated Akt is not inhibited by Aβ42. IP Akt1 from control and insulin-treated C2C12 myotubes in direct in vitro kinase assay. Insulin treatment before Akt1 IP showed expected increases in activity and phosphorylation, but was not inhibited by a range of Aβ42 doses (lanes 4–6). R, reverse peptide (42-1); F,Aβ1-42. (B) Preactivated Akt is dephosphorylated and inhibited by PP2A. Aβ42 and scrambled Aβ42 had no effect on insulin-conditioned Akt1 activity, whereas PP2A (0.25 μg/500 μl) did, quantified below. (C) Extracellular Aβ has no effect on Akt signaling. C2C12 myotubes were bath-exposed to Aβ25-35, reverse Aβ35-25, Aβ1-42, and reverse Aβ42-1 peptides for 24 h (or shorter times, not shown) before insulin (0.1 μg/ml, 15 min) treatments. Levels of endogenous p-Akt (Ser473), total Akt, p-GSK-3α/β, and total GSK-3β in whole cell extracts remain unchanged in the presence of extracellular Aβ. Insulin stimulation significantly increased levels of Akt activation (lane 2). (D) Insulin responsiveness unaffected by extracellular Aβ. C2C12 myotube cultures were exposed (15 min) to increasing doses of insulin at [Aβ1-42] = 0, 10, and 25 μM (2 h). Harvested cell extracts were fractionated by PAGE (10%) and developed for p-Akt (Thr308). (E) Phosphorylation status of PDK (pSer241). Insulin treatment did not further increase constitutively phosphorylated PDK (Ser241; top and bottom, lanes 1 and 2). Myotube cultures were exposed to insulin (500 ng/ml, 30 min) after either 2- or 24-h treatments of Aβ42 (1–100 nM).
Figure 7.
Aβi expression and PI3K activity. (A) PI3K assay. IP PI3K was added to PI in the presence of [γ-32P]ATP. Phopholipids were extracted and separated by TLC. Below, results quantified over time. (B) PI3K activity maintained in the presence of Aβ. PI3K assay in the presence of the inhibitor LY294002 (LY, 100 μM), Aβ42-1 (5 μM), or increasing Aβ1-42 (0.05, 0.5, and 5 μM). (C) Dephosphorylation of PI3P by phospholipase A2 (PLA2) but not Aβ42. PLA2 (500 μg/ml) or Aβ42 (10 μM) were added just before extraction in CHCl3/methanol. (D) PTEN, but not Aβ, inhibits PI3K. PI3K activity was determined in the presence of PTEN (10 μg/ml) or Aβ42 (10 μM). The phosphatase inhibited PI3K activity ∼50%; Aβ42 (10 μM) had no effect. Scrambled peptide (Scr; 10 μM) is control.
Figure 8.
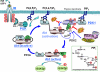
Model of insulin-PI3K-Akt signaling in AD. Intracellular Aβ is depicted to interfere with Akt activation by PDK-1 via two possible mechanisms. This may occur at a possible hydrophobic interaction site between Akt and PDK or directly through inhibition of the ATP-dependent kinase's ability to phosphorylate T308 of Akt. PH, pleckstrin homology domain; RD, regulatory domain; KD, kinase domain; G, growth factor (insulin; IGF-I); IR, insulin receptor; IRS-1, insulin receptor substrate-1; PI3K, phosphoinositide 3′ kinase; PIP3, PI(3,4,5)P3 (phosphoinositide phosphate, see inset for details); mTOR, mammalian target of rapamycin; , phosphorylated residues; ●, phosphorylated inositide (see inset).
, phosphorylated residues; ●, phosphorylated inositide (see inset).
Similar articles
- mTORC2 (Rictor) in Alzheimer's Disease and Reversal of Amyloid-β Expression-Induced Insulin Resistance and Toxicity in Rat Primary Cortical Neurons.
Lee HK, Kwon B, Lemere CA, de la Monte S, Itamura K, Ha AY, Querfurth HW. Lee HK, et al. J Alzheimers Dis. 2017;56(3):1015-1036. doi: 10.3233/JAD-161029. J Alzheimers Dis. 2017. PMID: 28035937 Free PMC article. - Age-dependent accumulation of soluble amyloid beta (Abeta) oligomers reverses the neuroprotective effect of soluble amyloid precursor protein-alpha (sAPP(alpha)) by modulating phosphatidylinositol 3-kinase (PI3K)/Akt-GSK-3beta pathway in Alzheimer mouse model.
Jimenez S, Torres M, Vizuete M, Sanchez-Varo R, Sanchez-Mejias E, Trujillo-Estrada L, Carmona-Cuenca I, Caballero C, Ruano D, Gutierrez A, Vitorica J. Jimenez S, et al. J Biol Chem. 2011 May 27;286(21):18414-25. doi: 10.1074/jbc.M110.209718. Epub 2011 Apr 1. J Biol Chem. 2011. PMID: 21460223 Free PMC article. - Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice.
Wang C, Zhang X, Teng Z, Zhang T, Li Y. Wang C, et al. Eur J Pharmacol. 2014 Oct 5;740:312-20. doi: 10.1016/j.ejphar.2014.06.051. Epub 2014 Jul 17. Eur J Pharmacol. 2014. PMID: 25041840 - Brain insulin resistance in Down syndrome: Involvement of PI3K-Akt/mTOR axis in early-onset of Alzheimer's disease and its potential as a therapeutic target.
Azimzadeh M, Cheah PS, Ling KH. Azimzadeh M, et al. Biochem Biophys Res Commun. 2024 Nov 12;733:150713. doi: 10.1016/j.bbrc.2024.150713. Epub 2024 Sep 17. Biochem Biophys Res Commun. 2024. PMID: 39307112 Review. - Implications of Phosphoinositide 3-Kinase-Akt (PI3K-Akt) Pathway in the Pathogenesis of Alzheimer's Disease.
Kumar M, Bansal N. Kumar M, et al. Mol Neurobiol. 2022 Jan;59(1):354-385. doi: 10.1007/s12035-021-02611-7. Epub 2021 Oct 26. Mol Neurobiol. 2022. PMID: 34699027 Review.
Cited by
- Age-induced nitrative stress decreases retrograde transport of proNGF via TrkA and increases proNGF retrograde transport and neurodegeneration via p75NTR.
Kropf E, Shekari A, Jaberi S, Puri A, Wu C, Fahnestock M. Kropf E, et al. Front Mol Neurosci. 2023 Nov 13;16:1241420. doi: 10.3389/fnmol.2023.1241420. eCollection 2023. Front Mol Neurosci. 2023. PMID: 38025269 Free PMC article. - Using Optogenetics to Model Cellular Effects of Alzheimer's Disease.
Tiwari P, Tolwinski NS. Tiwari P, et al. Int J Mol Sci. 2023 Feb 21;24(5):4300. doi: 10.3390/ijms24054300. Int J Mol Sci. 2023. PMID: 36901729 Free PMC article. Review. - Phosphorylation and Dephosphorylation of Beta-Amyloid Peptide in Model Cell Cultures: The Role of Cellular Protein Kinases and Phosphatases.
Barykin EP, Yanvarev DV, Strelkova MA, Valuev-Elliston VT, Varshavskaya KB, Mitkevich VA, Makarov AA. Barykin EP, et al. Life (Basel). 2023 Jan 4;13(1):147. doi: 10.3390/life13010147. Life (Basel). 2023. PMID: 36676097 Free PMC article. - From Small Peptides to Large Proteins against Alzheimer'sDisease.
Picone P, Sanfilippo T, Vasto S, Baldassano S, Guggino R, Nuzzo D, Bulone D, San Biagio PL, Muscolino E, Monastero R, Dispenza C, Giacomazza D. Picone P, et al. Biomolecules. 2022 Sep 22;12(10):1344. doi: 10.3390/biom12101344. Biomolecules. 2022. PMID: 36291553 Free PMC article. Review. - Age-Related Oxidative Redox and Metabolic Changes Precede Intraneuronal Amyloid-β Accumulation and Plaque Deposition in a Transgenic Alzheimer's Disease Mouse Model.
Pontrello CG, McWhirt JM, Glabe CG, Brewer GJ. Pontrello CG, et al. J Alzheimers Dis. 2022;90(4):1501-1521. doi: 10.3233/JAD-220824. J Alzheimers Dis. 2022. PMID: 36278355 Free PMC article.
References
- Alessi D. R., Cohen P. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 1998;8:55–62. - PubMed
- Andjelkovic M., Alessi D. R., Meier R., Fernandez A., Lamb N. J., Frech M., Cron P., Cohen P., Lucocq J. M., Hemmings B. A. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 1997;272:31515–31524. - PubMed
- Asano T., Yao Y., Shin S., McCubrey J., Abbruzzese J. L., Reddy S. A. Insulin receptor substrate is a mediator of phosphoinositide 3-kinase activation in quiescent pancreatic cancer cells. Cancer Res. 2005;65:9164–9168. - PubMed
- Bondy C. A., Cheng C. M. Signaling by insulin-like growth factor 1 in brain. Eur. J. Pharmacol. 2004;490:25–31. - PubMed
- Brunet A., Datta S. R., Greenberg M. E. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr. Opin. Neurobiol. 2001;11:297–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical