Atypical antipsychotic drugs and the risk of sudden cardiac death - PubMed (original) (raw)
Atypical antipsychotic drugs and the risk of sudden cardiac death
Wayne A Ray et al. N Engl J Med. 2009.
Erratum in
- N Engl J Med. 2009 Oct 29;361(18):1814
Abstract
Background: Users of typical antipsychotic drugs have an increased risk of serious ventricular arrhythmias and sudden cardiac death. However, less is known regarding the cardiac safety of the atypical antipsychotic drugs, which have largely replaced the older agents in clinical practice.
Methods: We calculated the adjusted incidence of sudden cardiac death among current users of antipsychotic drugs in a retrospective cohort study of Medicaid enrollees in Tennessee. The primary analysis included 44,218 and 46,089 baseline users of single typical and atypical drugs, respectively, and 186,600 matched nonusers of antipsychotic drugs. To assess residual confounding related to factors associated with the use of antipsychotic drugs, we performed a secondary analysis of users of antipsychotic drugs who had no baseline diagnosis of schizophrenia or related psychoses and with whom nonusers were matched according to propensity score (i.e., the predicted probability that they would be users of antipsychotic drugs).
Results: Current users of typical and of atypical antipsychotic drugs had higher rates of sudden cardiac death than did nonusers of antipsychotic drugs, with adjusted incidence-rate ratios of 1.99 (95% confidence interval [CI], 1.68 to 2.34) and 2.26 (95% CI, 1.88 to 2.72), respectively. The incidence-rate ratio for users of atypical antipsychotic drugs as compared with users of typical antipsychotic drugs was 1.14 (95% CI, 0.93 to 1.39). Former users of antipsychotic drugs had no significantly increased risk (incidence-rate ratio, 1.13; 95% CI, 0.98 to 1.30). For both classes of drugs, the risk for current users increased significantly with an increasing dose. Among users of typical antipsychotic drugs, the incidence-rate ratios increased from 1.31 (95% CI, 0.97 to 1.77) for those taking low doses to 2.42 (95% CI, 1.91 to 3.06) for those taking high doses (P<0.001). Among users of atypical agents, the incidence-rate ratios increased from 1.59 (95% CI, 1 .03 to 2.46) for those taking low doses to 2.86 (95% CI, 2.25 to 3.65) for those taking high doses (P=0.01). The findings were similar in the cohort that was matched for propensity score.
Conclusions: Current users of typical and of atypical antipsychotic drugs had a similar, dose-related increased risk of sudden cardiac death.
2009 Massachusetts Medical Society
Figures
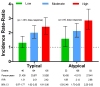
Figure 1
Adjusted incidence rate-ratio for sudden cardiac death among current users of antipsychotics, according to antipsychotic type and dose (chlorpromazine equivalents: low, <100mg; moderate, 100mg–299mg; high, ≥300mg). The reference category is that of nonusers of any antipsychotic drug. Vertical bars denote 95% confidence intervals.
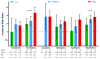
Figure 2
Adjusted incidence rate-ratio for sudden cardiac death among current users of six frequently prescribed individual antipsychotic drugs, according to dose( chlorpromazine equivalents: low, <100mg; moderate, 100mg–299mg; high, ≥300mg). The reference category is that of nonusers of any antipsychotic drug. Vertical bars denote 95% confidence intervals.
Comment in
- Antipsychotic agents and sudden cardiac death--how should we manage the risk?
Schneeweiss S, Avorn J. Schneeweiss S, et al. N Engl J Med. 2009 Jan 15;360(3):294-6. doi: 10.1056/NEJMe0809417. N Engl J Med. 2009. PMID: 19144946 No abstract available. - Atypical antipsychotic drugs and the risk of sudden cardiac death.
Baldessarini RJ. Baldessarini RJ. N Engl J Med. 2009 May 14;360(20):2136-7; author reply 2137-8. doi: 10.1056/NEJMc090291. N Engl J Med. 2009. PMID: 19439751 No abstract available. - Atypical antipsychotic drugs and the risk of sudden cardiac death.
Price LH. Price LH. N Engl J Med. 2009 May 14;360(20):2137; author reply 2137-8. N Engl J Med. 2009. PMID: 19445034 No abstract available. - Typical and atypical antipsychotics increase risk of sudden cardiac death.
Taylor D. Taylor D. Evid Based Ment Health. 2009 Aug;12(3):92. doi: 10.1136/ebmh.12.3.92. Evid Based Ment Health. 2009. PMID: 19633260 No abstract available. - ACP Journal Club. Both typical and atypical antipsychotic agents were associated with increased risk for sudden cardiac death.
Marder S. Marder S. Ann Intern Med. 2009 Aug 18;151(4):JC2-12. doi: 10.7326/0003-4819-151-4-200908180-02012. Ann Intern Med. 2009. PMID: 19687486 No abstract available.
Similar articles
- Antipsychotics and the risk of sudden cardiac death.
Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Ray WA, et al. Arch Gen Psychiatry. 2001 Dec;58(12):1161-7. doi: 10.1001/archpsyc.58.12.1161. Arch Gen Psychiatry. 2001. PMID: 11735845 - Risk of mortality (including sudden cardiac death) and major cardiovascular events in atypical and typical antipsychotic users: a study with the general practice research database.
Murray-Thomas T, Jones ME, Patel D, Brunner E, Shatapathy CC, Motsko S, Van Staa TP. Murray-Thomas T, et al. Cardiovasc Psychiatry Neurol. 2013;2013:247486. doi: 10.1155/2013/247486. Epub 2013 Dec 26. Cardiovasc Psychiatry Neurol. 2013. PMID: 24455199 Free PMC article. - Risk of death in dual-eligible nursing home residents using typical or atypical antipsychotic agents.
Aparasu RR, Chatterjee S, Mehta S, Chen H. Aparasu RR, et al. Med Care. 2012 Nov;50(11):961-9. doi: 10.1097/MLR.0b013e31826ec185. Med Care. 2012. PMID: 23047786 - The relationship between atypical antipsychotics drugs, QT interval prolongation, and torsades de pointes: implications for clinical use.
Ruiz Diaz JC, Frenkel D, Aronow WS. Ruiz Diaz JC, et al. Expert Opin Drug Saf. 2020 May;19(5):559-564. doi: 10.1080/14740338.2020.1745184. Epub 2020 Apr 15. Expert Opin Drug Saf. 2020. PMID: 32189527 Review. - Sudden Cardiac Death in Schizophrenia: A Review.
Vohra J. Vohra J. Heart Lung Circ. 2020 Oct;29(10):1427-1432. doi: 10.1016/j.hlc.2020.07.003. Epub 2020 Aug 3. Heart Lung Circ. 2020. PMID: 32800442 Review.
Cited by
- The effects of aripiprazole on electrocardiography in children with pervasive developmental disorders.
Ho JG, Caldwell RL, McDougle CJ, Orsagh-Yentis DK, Erickson CA, Posey DJ, Stigler KA. Ho JG, et al. J Child Adolesc Psychopharmacol. 2012 Aug;22(4):277-83. doi: 10.1089/cap.2011.0129. Epub 2012 Jul 31. J Child Adolesc Psychopharmacol. 2012. PMID: 22849533 Free PMC article. Clinical Trial. - Reply to "Antipsychotics: Mortality Risk Themselves?".
Herzig SJ, Marcantonio ER. Herzig SJ, et al. J Am Geriatr Soc. 2016 Nov;64(11):2400. doi: 10.1111/jgs.14506. Epub 2016 Oct 26. J Am Geriatr Soc. 2016. PMID: 27783402 Free PMC article. No abstract available. - Antipsychotics and Medical Comorbidity: A Retrospective Study in an Urban Outpatient Psychiatry Clinic.
Bennett CW, Gensler L, Goldsmith DR. Bennett CW, et al. Community Ment Health J. 2023 May;59(4):641-653. doi: 10.1007/s10597-022-01045-2. Epub 2022 Nov 10. Community Ment Health J. 2023. PMID: 36355255 - Psychotropic medications and the risk of sudden cardiac death.
Huikuri HV. Huikuri HV. J Am Heart Assoc. 2015 Feb 23;4(2):e001894. doi: 10.1161/JAHA.115.001894. J Am Heart Assoc. 2015. PMID: 25713295 Free PMC article. No abstract available. - Rise in mortality involving poisoning by medicaments other than narcotics, including poisoning by psychotropic drugs in different age/racial groups in the US.
Goldstein E. Goldstein E. PLoS One. 2019 Jul 19;14(7):e0219711. doi: 10.1371/journal.pone.0219711. eCollection 2019. PLoS One. 2019. PMID: 31323036 Free PMC article.
References
- Haddad PM, Anderson IM. Antipsychotic-related QTc prolongation, torsade de pointes and sudden death. Drugs. 2002;62:1649–1671. - PubMed
- Kongsamut S, Kang J, Chen XL, Roehr J, Rampe D. A comparison of the receptor binding and HERG channel affinities for a series of antipsychotic drugs. Eur J Pharmacol. 2002;450:37–41. - PubMed
- Thomas SHL. Drugs, QT interval abnormalities and ventricular arrhythmias. Adverse Drug React Toxicol Rev. 1994;13:77–102. - PubMed
- Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas HL. QTc- interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355:1048–1052. - PubMed
- Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. - PubMed
Reference List
- Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther. 2003;75:234–241. - PubMed
- Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein CM. Oral erythromycin use and the risk of sudden cardiac death. N Engl J Med. 2004;351:1089–1096. - PubMed
- Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacol Drug Safety. 2005;14:747–753. - PubMed
- Wiseman LR, Faulds D. Cisapride. An updated review of its pharmacology and therapeutic efficacy as a prokinetic agent in gastrointestinal motility disorders. Drugs. 1994;47:116–152. - PubMed
- Monahan BP, Ferguson CL, Killeavy ES, Lloyd BK, Troy J, Cantilena LR. Torsades de pointes occurring in association with terfenadine use. JAMA. 1990;264:2788–2790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources