Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates - PubMed (original) (raw)
. 2009 Feb;119(2):323-35.
doi: 10.1172/JCI32661. Epub 2009 Jan 19.
Affiliations
- PMID: 19147984
- PMCID: PMC2631287
- DOI: 10.1172/JCI32661
Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates
Carrie E McCurdy et al. J Clin Invest. 2009 Feb.
Abstract
Maternal obesity is thought to increase the offspring's risk of juvenile obesity and metabolic diseases; however, the mechanism(s) whereby excess maternal nutrition affects fetal development remain poorly understood. Here, we investigated in nonhuman primates the effect of chronic high-fat diet (HFD) on the development of fetal metabolic systems. We found that fetal offspring from both lean and obese mothers chronically consuming a HFD had a 3-fold increase in liver triglycerides (TGs). In addition, fetal offspring from HFD-fed mothers (O-HFD) showed increased evidence of hepatic oxidative stress early in the third trimester, consistent with the development of nonalcoholic fatty liver disease (NAFLD). O-HFD animals also exhibited elevated hepatic expression of gluconeogenic enzymes and transcription factors. Furthermore, fetal glycerol levels were 2-fold higher in O-HFD animals than in control fetal offspring and correlated with maternal levels. The increased fetal hepatic TG levels persisted at P180, concurrent with a 2-fold increase in percent body fat. Importantly, reversing the maternal HFD to a low-fat diet during a subsequent pregnancy improved fetal hepatic TG levels and partially normalized gluconeogenic enzyme expression, without changing maternal body weight. These results suggest that a developing fetus is highly vulnerable to excess lipids, independent of maternal diabetes and/or obesity, and that exposure to this may increase the risk of pediatric NAFLD.
Figures
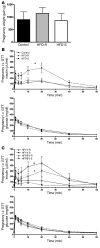
Figure 1. HFD-fed pregnant female macaques display hyperinsulinemia and hyperglycerolemia independent of weight gain.
All data in this figure were obtained from animals at G130. (A) Weight gain during pregnancy was similar in all 3 groups. Data were analyzed using 1-way ANOVA with Bonferroni post-hoc analysis. (B) Insulin secretion and glucose clearance during i.v. GTT for control, HFD-R, and HFD-S groups. The HFD groups are from animals fed HFD for 2–4 years and were obtained during the early third trimester. *P < 0.05 versus control. (A and B) n = 17 (control); 10 (HFD-S); 8 (HFD-R). (C) Insulin secretion and glucose clearance during i.v. GTT for HFY4 and HFREV groups. #P < 0.05 versus HFY4-R; †P < 0.05 versus HFY4-S, 2-tailed Student’s t test. n = 3 (HFY4-R and HFREV-R); 4 (HFY4-S and HFREV-S).
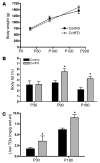
Figure 2. Maternal HFD feeding during pregnancy and the perinatal period increases offspring adiposity and liver TG levels.
(A) Body weights of O-HFD and control offspring at P30, P90, and P180. There was no statistical difference between the groups at any age. (B) Whole body fat composition, as determined by DEXA analysis, at all 3 ages in both groups. (C) Liver TG levels in O-HFD and control offspring at P30 and P180. For TG analysis of P30 offspring, n = 4 for both control and O-HFD (2 male and 2 female). Liver samples were obtained from these offspring at the time of termination. A separate group of animals was used to obtain the serial data in A and B and the P180 liver TGs in C. The control group consisted of 4 animals (2 male and 2 female), while the O-HFD group consisted of 7 animals (4 male and 3 female). Liver samples were obtained from these animals by biopsies obtained through laparoscopic surgery. *P < 0.05 versus respective control, 2-tailed Student’s t test.
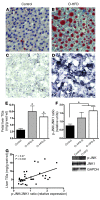
Figure 3. Maternal HFD feeding leads to lipid accumulation and activation of oxidative stress in the fetal liver.
(A–D) Oil Red O staining (A and B) and HNE staining (C and D) in the fetal livers of controls (A and C) and O-HFD animals (B and D). Original magnification, ×20. (E) Fetal liver TGs were significantly elevated in both O–HFD-R and O–HFD-S groups. *P < 0.05 versus control (1-way ANOVA with Bonferroni post-hoc analysis). (F) As a group, O-HFD animals had a significant increase in the ratio of p-JNK1 to total JNK1 levels. Representative Western blots are also shown. *P < 0.05 (2-tailed Student’s t test). (G) There was a significant correlation between TG levels and the ratio of p-JNK1 to total JNK1 in the fetal livers. n = 6 (O–HFD-R); 9 (control and O–HFD-S).
Figure 5. Maternal diet reversal partially prevents the development of fatty liver in the fetal offspring.
(A) Liver TG levels for O-HFY4 and O-HFREV animals. Dashed line represents TG levels in normal control fetal offspring. #P < 0.05 versus O-HFY4 (2-tailed Student’s t test). (B–E) Relative difference in G6P (B), FBP1 (C), PCK1 (D), and PGC1A (E) expression in the fetal liver of control (n = 9), O-HFD (n = 15), and O-HFREV (n = 7) groups. Data were obtained from samples obtained at different times and run in different assays, but were normalized to control samples that were present in every assay. O-HFD values represent the average of both O–HFD-R and O–HFD-S groups from Figure 4. *P < 0.05 versus control (ANOVA with Bonferonni’s multiple comparison test). There was no significant difference between the O-HFREV and either the control or the O-HFD groups.
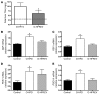
Figure 4. Maternal HFD feeding leads to increased expression of genes in the gluconeogenic pathway in the fetal liver.
(A–D) As a group, O-HFD fetuses had significantly elevated levels of G6P (A), FBP1 (B), PCK1 (C), and PGC1A (D) mRNA in the liver. *P < 0.05 (2-tailed Student’s t test). (E) PCK1 mRNA expression significantly correlated with fetal liver TG levels. (F) The ratio of nuclear acetylated PGC1α (acPGC1α) to total PGC1α protein was significantly decreased in both O–HFD-R and O–HFD-S groups. *P < 0.05 versus control (1-way ANOVA with Bonferroni post-hoc analysis). (G) Representative Western blot of acetylated PGC1α. (H) As a group, O-HFD animals had significantly elevated HNF4α protein levels in the fetal liver. *P < 0.05 (2-tailed Student’s t test). (I) Representative Western blot of HNF4α. n = 6 (O–HFD-R); 9 (control and O–HFD-S).
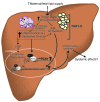
Figure 6. Effects of increased maternal/fetal lipid supply on hepatic lipotoxicity in the fetus.
An increase in the availability of lipids to the fetus during early development prior to adipose tissue accretion results in lipid accumulation in nonadipose fetal tissues, leading to toxic effects. In O-HFD fetal liver, there was a significant increase in fetal hepatic TG accumulation that correlated with increased JNK activation. Liver staining revealed substantial elevations in markers of oxidative stress and damage in O-HFD livers. It is likely that the elevated TG levels lead to macrophage infiltration and contribute to the liver inflammation. Liver TG accumulation and inflammation are important events underlying NAFLD. The elevation in liver stress activates heat shock proteins (HSPs), which have previously been shown to be involved in chromatin remodeling (epigenetic programming; ref. 98). Oxidative stress and JNK activation triggers transcription for genes involved in gluconeogenesis as well as inflammation. Upregulation of inflammatory cytokines likely contributes to the progression of NAFLD, while early activation of gluconeogenic genes, such as PCK1, in fetal liver may predispose offspring for increased hepatic gluconeogenesis and insulin resistance.
Similar articles
- Switching obese mothers to a healthy diet improves fetal hypoxemia, hepatic metabolites, and lipotoxicity in non-human primates.
Wesolowski SR, Mulligan CM, Janssen RC, Baker PR 2nd, Bergman BC, D'Alessandro A, Nemkov T, Maclean KN, Jiang H, Dean TA, Takahashi DL, Kievit P, McCurdy CE, Aagaard KM, Friedman JE. Wesolowski SR, et al. Mol Metab. 2018 Dec;18:25-41. doi: 10.1016/j.molmet.2018.09.008. Epub 2018 Sep 28. Mol Metab. 2018. PMID: 30337225 Free PMC article. - Maternal high fat diet is associated with decreased plasma n-3 fatty acids and fetal hepatic apoptosis in nonhuman primates.
Grant WF, Gillingham MB, Batra AK, Fewkes NM, Comstock SM, Takahashi D, Braun TP, Grove KL, Friedman JE, Marks DL. Grant WF, et al. PLoS One. 2011 Feb 25;6(2):e17261. doi: 10.1371/journal.pone.0017261. PLoS One. 2011. PMID: 21364873 Free PMC article. - Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates.
Thorn SR, Baquero KC, Newsom SA, El Kasmi KC, Bergman BC, Shulman GI, Grove KL, Friedman JE. Thorn SR, et al. Diabetes. 2014 Aug;63(8):2702-13. doi: 10.2337/db14-0276. Epub 2014 Apr 4. Diabetes. 2014. PMID: 24705404 Free PMC article. - Developmental origins of nonalcoholic fatty liver disease.
Brumbaugh DE, Friedman JE. Brumbaugh DE, et al. Pediatr Res. 2014 Jan;75(1-2):140-7. doi: 10.1038/pr.2013.193. Epub 2013 Nov 5. Pediatr Res. 2014. PMID: 24192698 Free PMC article. Review. - Mitochondrial role in the neonatal predisposition to developing nonalcoholic fatty liver disease.
Baker PR 2nd, Friedman JE. Baker PR 2nd, et al. J Clin Invest. 2018 Aug 31;128(9):3692-3703. doi: 10.1172/JCI120846. Epub 2018 Aug 31. J Clin Invest. 2018. PMID: 30168806 Free PMC article. Review.
Cited by
- Epigenetic and transgenerational reprogramming of brain development.
Bale TL. Bale TL. Nat Rev Neurosci. 2015 Jun;16(6):332-44. doi: 10.1038/nrn3818. Epub 2015 Apr 29. Nat Rev Neurosci. 2015. PMID: 25921815 Free PMC article. Review. - Maternal Weight Intervention in the Perinatal Period Improves Liver Health in the Offspring of Mothers with Obesity.
Purcell AR, Rodrigo N, Cao Q, Joseph O, Gill AJ, Saad S, Pollock CA, Glastras SJ. Purcell AR, et al. Nutrients. 2023 Dec 28;16(1):109. doi: 10.3390/nu16010109. Nutrients. 2023. PMID: 38201940 Free PMC article. - A maternal high-fat diet modulates fetal SIRT1 histone and protein deacetylase activity in nonhuman primates.
Suter MA, Chen A, Burdine MS, Choudhury M, Harris RA, Lane RH, Friedman JE, Grove KL, Tackett AJ, Aagaard KM. Suter MA, et al. FASEB J. 2012 Dec;26(12):5106-14. doi: 10.1096/fj.12-212878. Epub 2012 Sep 14. FASEB J. 2012. PMID: 22982377 Free PMC article. - Pediatric Non-Alcoholic Fatty Liver Disease: Nutritional Origins and Potential Molecular Mechanisms.
Mandala A, Janssen RC, Palle S, Short KR, Friedman JE. Mandala A, et al. Nutrients. 2020 Oct 16;12(10):3166. doi: 10.3390/nu12103166. Nutrients. 2020. PMID: 33081177 Free PMC article. Review. - α-Melanocyte stimulating hormone promotes muscle glucose uptake via melanocortin 5 receptors.
Enriori PJ, Chen W, Garcia-Rudaz MC, Grayson BE, Evans AE, Comstock SM, Gebhardt U, Müller HL, Reinehr T, Henry BA, Brown RD, Bruce CR, Simonds SE, Litwak SA, McGee SL, Luquet S, Martinez S, Jastroch M, Tschöp MH, Watt MJ, Clarke IJ, Roth CL, Grove KL, Cowley MA. Enriori PJ, et al. Mol Metab. 2016 Aug 5;5(10):807-822. doi: 10.1016/j.molmet.2016.07.009. eCollection 2016 Oct. Mol Metab. 2016. PMID: 27688995 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- DK-60685/DK/NIDDK NIH HHS/United States
- R01 DK062155/DK/NIDDK NIH HHS/United States
- P51 RR000163/RR/NCRR NIH HHS/United States
- K01 RR000163/RR/NCRR NIH HHS/United States
- DK-062155/DK/NIDDK NIH HHS/United States
- DK-79194/DK/NIDDK NIH HHS/United States
- R01 DK060685/DK/NIDDK NIH HHS/United States
- R01 HD014643/HD/NICHD NIH HHS/United States
- DKF32-075252/PHS HHS/United States
- R01 DK079194/DK/NIDDK NIH HHS/United States
- RR00163/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous