PPAR-gamma AF-2 domain functions as a component of a ubiquitin-dependent degradation signal - PubMed (original) (raw)
PPAR-gamma AF-2 domain functions as a component of a ubiquitin-dependent degradation signal
Gail E Kilroy et al. Obesity (Silver Spring). 2009 Apr.
Abstract
The nuclear hormone receptor peroxisome proliferator-activated receptor-gamma (PPAR-gamma) functions as the "master switch" in adipocyte development and is important in regulating glucose metabolism. PPAR-gamma is rapidly degraded in adipocytes by the ubiquitin proteasome pathway under basal and ligand-activated conditions. Proteasome inhibition increases PPAR-gamma activity, indicating disposal of PPAR-gamma by the ubiquitin proteasome system regulates PPAR-gamma activity. However, the signals and factors required for recognition of PPAR-gamma by the ubiquitin proteasome pathway are unknown. To begin understanding how the ubiquitin-proteasome pathway interacts with PPAR-gamma, we designed a series of constructs containing each PPAR-gamma domain expressed as a fusion protein with the GAL4 DNA-binding domain. The ability of each PPAR-gamma domain to alter the stability of the GAL4 DNA-binding domain and to undergo ubiquitylation was assessed via western blot analysis. In addition, luciferase reporter assays were used to assay PPAR-gamma transcriptional activity. Using this approach, we determined that the AF-1 and ligand-binding domains (LBDs) of PPAR-gamma are targeted to the proteasome for degradation. However, only the LBD is conjugated to ubiquitin. The AF-2 helix of the LBD is required for maximum ubiquitylation, but is not essential for ligand-dependent ubiquitin conjugation. Finally, luciferase reporter assays show a fully functional ubiquitin system is required for PPAR-gamma activation. These results indicate that the ubiquitin-proteasome pathway is an integral determinant of PPAR-gamma activity, targeting PPAR-gamma for proteasomal degradation via ubiquitin independent and ubiquitin dependent mechanisms.
Figures
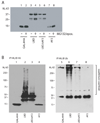
Figure 1. Ubiquitylation regulates PPARγ activity
(A) NIH 3T3 fibroblast cells were transiently transfected with PPREx3-TK-luciferase in the absence or presence of an expression vector for wild-type PPARγ2. Forty-eight hours later, the cells were treated with a ubiquitin activating enzyme (E1) inhibitor (E1-I, 50 µM) or DMSO as a vehicle control. After one hour, rosiglitazone (TZD, 5 µM) was added alone or in combination with the E1 inhibitor as indicated. Six hours thereafter, cell lysates were obtained and analyzed for luciferase activity. PPARγ2 activity is reported in relative light units (RLU)/µg protein as fold increase over luciferase alone plus TZD. (B) NIH-3T3 cells were transfected with PPREx3-TK-luciferase and wild-type PPARγ2 along with either pcDNA3.1 (+), wild-type ubiquitin, or ubiquitin K48R, G76A expression vectors as indicated. Forty-eight hours later, the cells were treated with rosiglitazone (TZD, 5 µM) for six hours. Whole cell extracts were then harvested and assayed for luciferase activity. PPARγ2 activity is reported as fold increase over PPARγ2 with pcDNA 3.1(+) in the absence of TZD. An equal amount of DNA was transfected into the cells with empty vector (pcDNA 3.1) used to balance the total amount of DNA in each case. Each experiment was independently performed three times and a representative experiment is shown. (C, D) Fully differentiated 3T3-L1 adipocytes were pretreated with E1-I (50 µM) or MG132 (10 µM) and epoxomicin (0.1 µM) for one hour prior to the addition of rosiglitazone (TZD, 5 µM). (C) Whole cell extracts were harvested at the end of four hours and twenty-five µg of each extract were separated by SDS-PAGE and subjected to western blot analysis. (D) Whole cell extracts were harvested at the end of one hour and one mg of each extract was immunoprecipitated using anti-PPARγ antibody, separated by SDS-PAGE, and subjected to western blot analysis using either anti-PPARγ antibody (IP: PPARγ, IB: PPARγ) or anti-ubiquitin antibody (IP: PPARγ, IB: ubiquitin). The experiments in (C) and (D) were performed independently twice.
Figure 2. GAL4DBD-HA-PPARγ domain fusion proteins
(A) PPARγ2 is primarily expressed in adipocytes and differs from PPARγ1 by a N-terminal 30 amino acid extension. Both forms of PPARγ contain 36 lysines. (B) GAL4-HA-PPARγ fusion proteins used in this study. GAL4 DBD-HA, GAL4 DNA binding domain fused to a hemagglutinin (HA) epitope tag; AF-1, Activation Function 1 domain; DBD, DNA binding domain; LBD, Ligand binding domain; LBDΔAF-2, deletion of the activation function 2 domain (helix 12) from the LBD; AF-2, activation function 2 domain only.
Figure 3. PPARγ AF-1 and ligand binding domains are recognized by the proteasome
An equal amount of cDNA encoding each GAL4DBD-HA-PPARγ fusion protein was transiently transfected into 3T3-L1 preadipocytes. GAL4-DBD-HA alone is indicated as GAL4HA and the GAL4DBD-HA-PPARγ domain fusion proteins are indicated by the PPARγ domain. Forty-eight hours after transfection, the cells were incubated in the absence (DMSO as vehicle control) or presence of MG132 (10 µM) and epoxomicin (0.1 µM) for 1 hour. Whole cell extracts were harvested and equal protein amounts were separated by SDS-PAGE followed by transfer to nitrocellulose. Western blot analysis was carried out with an anti-HA monoclonal antibody followed by chemiluminence detection of a horseradish peroxidase conjugated secondary antibody. (A) is a short film exposure and (B) is a longer film exposure of independent experiments.
Figure 4. PPARγ ligand binding domain is conjugated to ubiquitin
NIH 3T3 fibroblasts were transiently transfected with an equal amount of cDNA encoding each GAL4DBD-HA-PPARγ fusion protein. GAL4-DBD-HA alone is indicated as GAL4HA and the GAL4DBD-HA-PPARγ domain fusion proteins are indicated by the PPARγ domain. Forty-eight hours after transfection, the cells were treated with MG132 (10 µM) and epoxomicin (0.1 µM) for 1 hour. Thereafter, whole cell extracts were harvested and immunoprecipitations were carried out using anti-HA antibody. Western blot analysis was performed using either (A) anti-HA (IP HA, IB HA) or (B) anti-ubiquitin (IP HA, IB Ub) primary antibodies.
Figure 5. The AF-2 domain partially overlaps the PPARγ LBD degron
(A) NIH 3T3 cells were transiently transfected with equal amounts of cDNA encoding the GAL4DBD-HA-PPARγ domain proteins. GAL4-DBD-HA alone is indicated as GAL4HA and the GAL4DBD-HA-PPARγ domain fusion proteins are indicated by the PPARγ domain. (A) Forty-eight hours later, the cells were incubated in the absence (DMSO) or presence of MG132 (10 µM) and epoxomicin (0.1 µM) for 1 hour. Whole cell extracts were harvested and western blot analysis was carried out using anti-HA primary antibody. (B) The cells were incubated for 1 hour with MG132 (10 µM) and epoxomicin (0.1 µM) prior to harvesting the whole cell extracts. Immunoprecipitations were carried out with anti-HA antibody followed by western blot analysis using either anti-HA (IP HA, IB HA) or anti-Ub (IP HA, IB Ub) primary antibodies.
Figure 6. Ligand dependent ubiquitylation of the PPARγ LBD does not require the AF-2 domain
NIH 3T3 cells were transiently transfected with cDNA encoding GAL4DBD-HA, GAL4DBD-HA-LBD, or GAL4DBD-HA-LBDΔAF-2 proteins. (A) Forty-eight hours later, the cells were treated with MG132 (10 µM) and epoxomicin (1 µM) for 1 hour followed by the addition of a vehicle control (DMSO) or rosiglitazone (TZD, 5 µM). Three hours later, the cells were harvested and immunoprecipitated using anti-HA primary antibody and subjected to western blot analysis using anti-ubiquitin antibody (IP HA, IB Ub). (B) A cDNA encoding SRC-1 was cotransfected with the GAL4-HA-LBD and GAL4-HA-LBDΔAF-2. Forty eight hours later, the cells were treated with rosiglitazone (TZD, 5 µM) for three hours. Whole cell extracts were harvested and immunoblotted with either anti-HA (IB HA) or anti-SRC-1 primary antibodies (IB SRC-1) directly or following immunoprecipitation using anti-HA primary antibody.
Similar articles
- Regulation of peroxisome proliferator-activated receptor gamma activity by losartan metabolites.
Schupp M, Lee LD, Frost N, Umbreen S, Schmidt B, Unger T, Kintscher U. Schupp M, et al. Hypertension. 2006 Mar;47(3):586-9. doi: 10.1161/01.HYP.0000196946.79674.8b. Epub 2005 Dec 19. Hypertension. 2006. PMID: 16365190 - Aryl hydrocarbon receptor (AhR) regulates adipocyte differentiation by assembling CRL4B ubiquitin ligase to target PPARγ for proteasomal degradation.
Dou H, Duan Y, Zhang X, Yu Q, Di Q, Song Y, Li P, Gong Y. Dou H, et al. J Biol Chem. 2019 Nov 29;294(48):18504-18515. doi: 10.1074/jbc.RA119.009282. Epub 2019 Oct 25. J Biol Chem. 2019. PMID: 31653699 Free PMC article. - Proteasome inhibitors induce peroxisome proliferator-activated receptor transactivation through RXR accumulation and a protein kinase C-dependent pathway.
Tsao WC, Wu HM, Chi KH, Chang YH, Lin WW. Tsao WC, et al. Exp Cell Res. 2005 Mar 10;304(1):234-43. doi: 10.1016/j.yexcr.2004.11.004. Epub 2004 Dec 10. Exp Cell Res. 2005. PMID: 15707588 - Control of peroxisome proliferator-activated receptor fate by the ubiquitinproteasome system.
Genini D, Catapano CV. Genini D, et al. J Recept Signal Transduct Res. 2006;26(5-6):679-92. doi: 10.1080/10799890600928202. J Recept Signal Transduct Res. 2006. PMID: 17118805 Review. - [Molecular mechanism of switching adipocyte / osteoblast differentiation through regulation of PPAR-gamma function].
Takada I, Kato S. Takada I, et al. Clin Calcium. 2008 May;18(5):656-61. Clin Calcium. 2008. PMID: 18445885 Review. Japanese.
Cited by
- Selective PPAR-Delta/PPAR-Gamma Activation Improves Cognition in a Model of Alzheimer's Disease.
Steinke I, Govindarajulu M, Pinky PD, Bloemer J, Yoo S, Ward T, Schaedig T, Young T, Wibowo FS, Suppiramaniam V, Amin RH. Steinke I, et al. Cells. 2023 Apr 8;12(8):1116. doi: 10.3390/cells12081116. Cells. 2023. PMID: 37190025 Free PMC article. - Modulation of the transcriptional activity of peroxisome proliferator-activated receptor gamma by protein-protein interactions and post-translational modifications.
Kim TH, Kim MY, Jo SH, Park JM, Ahn YH. Kim TH, et al. Yonsei Med J. 2013 May 1;54(3):545-59. doi: 10.3349/ymj.2013.54.3.545. Yonsei Med J. 2013. PMID: 23549795 Free PMC article. Review. - ACSS2 controls PPARγ activity homeostasis to potentiate adipose-tissue plasticity.
Chen N, Zhao M, Wu N, Guo Y, Cao B, Zhan B, Li Y, Zhou T, Zhu F, Guo C, Shi Y, Wang Q, Li Y, Zhang L. Chen N, et al. Cell Death Differ. 2024 Apr;31(4):479-496. doi: 10.1038/s41418-024-01262-0. Epub 2024 Feb 8. Cell Death Differ. 2024. PMID: 38332049 Free PMC article. - Controlling a master switch of adipocyte development and insulin sensitivity: covalent modifications of PPARγ.
Floyd ZE, Stephens JM. Floyd ZE, et al. Biochim Biophys Acta. 2012 Jul;1822(7):1090-5. doi: 10.1016/j.bbadis.2012.03.014. Epub 2012 Apr 4. Biochim Biophys Acta. 2012. PMID: 22504298 Free PMC article. Review. - High efficiency lipid-based siRNA transfection of adipocytes in suspension.
Kilroy G, Burk DH, Floyd ZE. Kilroy G, et al. PLoS One. 2009 Sep 11;4(9):e6940. doi: 10.1371/journal.pone.0006940. PLoS One. 2009. PMID: 19759827 Free PMC article.
References
- Tontonoz P, Spiegelman BM. Fat and Beyond: The Diverse Biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. - PubMed
- Hummasti S, Tontonoz P. The peroxisome proliferator-activated receptor N-terminal domain controls isotype-selective gene expression and adipogenesis. Mol Endocrinol. 2006;20:1261–1275. - PubMed
- Medina-Gomez G, Gray SL, Yetukuri L, Shimomura K, Virtue S, Campbell M, Curtis RK, Jimenez-Linan M, Blount M, Yeo GS, Lopez M, Seppanen-Laakso T, Ashcroft FM, Oresic M, Vidal-Puig A. PPAR gamma 2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3:e64. - PMC - PubMed
- Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR021945/RR/NCRR NIH HHS/United States
- P30 DK072476/DK/NIDDK NIH HHS/United States
- P20 RR021945-036641/RR/NCRR NIH HHS/United States
- R03 AG025751-02/AG/NIA NIH HHS/United States
- R03 AG025751/AG/NIA NIH HHS/United States
- R56 DK089020/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources