Complete genome sequence of the aerobic CO-oxidizing thermophile Thermomicrobium roseum - PubMed (original) (raw)
doi: 10.1371/journal.pone.0004207. Epub 2009 Jan 16.
Jason Raymond, Martin Wu, Sourav Chatterji, Qinghu Ren, Joel E Graham, Donald A Bryant, Frank Robb, Albert Colman, Luke J Tallon, Jonathan H Badger, Ramana Madupu, Naomi L Ward, Jonathan A Eisen
Affiliations
- PMID: 19148287
- PMCID: PMC2615216
- DOI: 10.1371/journal.pone.0004207
Complete genome sequence of the aerobic CO-oxidizing thermophile Thermomicrobium roseum
Dongying Wu et al. PLoS One. 2009.
Abstract
In order to enrich the phylogenetic diversity represented in the available sequenced bacterial genomes and as part of an "Assembling the Tree of Life" project, we determined the genome sequence of Thermomicrobium roseum DSM 5159. T. roseum DSM 5159 is a red-pigmented, rod-shaped, Gram-negative extreme thermophile isolated from a hot spring that possesses both an atypical cell wall composition and an unusual cell membrane that is composed entirely of long-chain 1,2-diols. Its genome is composed of two circular DNA elements, one of 2,006,217 bp (referred to as the chromosome) and one of 919,596 bp (referred to as the megaplasmid). Strikingly, though few standard housekeeping genes are found on the megaplasmid, it does encode a complete system for chemotaxis including both chemosensory components and an entire flagellar apparatus. This is the first known example of a complete flagellar system being encoded on a plasmid and suggests a straightforward means for lateral transfer of flagellum-based motility. Phylogenomic analyses support the recent rRNA-based analyses that led to T. roseum being removed from the phylum Thermomicrobia and assigned to the phylum Chloroflexi. Because T. roseum is a deep-branching member of this phylum, analysis of its genome provides insights into the evolution of the Chloroflexi. In addition, even though this species is not photosynthetic, analysis of the genome provides some insight into the origins of photosynthesis in the Chloroflexi. Metabolic pathway reconstructions and experimental studies revealed new aspects of the biology of this species. For example, we present evidence that T. roseum oxidizes CO aerobically, making it the first thermophile known to do so. In addition, we propose that glycosylation of its carotenoids plays a crucial role in the adaptation of the cell membrane to this bacterium's thermophilic lifestyle. Analyses of published metagenomic sequences from two hot springs similar to the one from which this strain was isolated, show that close relatives of T. roseum DSM 5159 are present but have some key differences from the strain sequenced.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
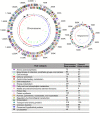
Figure 1. The chromosome and the megaplasmid of T. roseum.
The circles display the following features, starting with the outermost circle: (1) forward strand genes; (2) reverse strand genes; (3) chi square deviation of local nucleotide composition from the genome average; (4) GC skew (blue bars represent positive values, red bars represent negative values); (5) tRNAs (green lines); (6) rRNAs (blue lines); (7) small RNAs (red lines). Gene color indicates the assigned role category. A gene can be included in the gene count for multiple role categories.
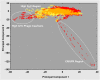
Figure 2. Analysis of nucleotide composition variations within the T. roseum chromosome and megaplasmid.
Hexamer frequencies were extracted from 20,000 random 4 kb fragments from the whole genome and analyzed using CompostBin, a PCA-based method. The first two principal components of the data are plotted. Red indicates chromosome data; yellow indicates megaplasmid data; orange indicates overlaps between the two; circles indicate the three outlier regions.
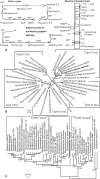
Figure 3. Maximum-likelihood tree of Thermomicrobium roseum and other bacterial species for which complete genomes are available.
The tree was built from concatenated alignments of 31 housekeeping genes using the PHYML program. The bootstrap values are based on 100 replications. Organisms with photosynthetic capability are emphasized in green. Members from the phylum Chloroflexi are highlighted by the shaded box. Note – the organism Chlorobaculum tepidum used to be known as Chlorobium tepidum.
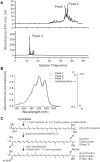
Figure 4. Carotenoid modification in T. roseum.
A. Reverse phase HPLC analysis of T. roseum pigments revealed two types of carotenoid compounds: extremely hydrophobic pigments from crude extracts (top panel, peaks 1 & 2) and a polar glycoside released by saponification (bottom panel, peak 3). The glycoside is the major form of carotenoid in T. roseum. Its HPLC retention time was similar to that of the authentic oscillaxanthin (oscillol-2,2′-difucoside) from the cyanobacterium Gloeobacter violaceus PCC7421 (see Figure 4B for the molecular structure). B. Comparison of the in-line spectra of compounds from peak 1, 2 & 3 (Figure 4A) with that of the oscillaxanthin from Gloeobacter violaceus PCC 7421 indicates that the major T. roseum carotenoid core is oscillol diglycoside. The structure of oscillaxanthin is shown to the right. The sugar moieties in oscillaxanthin are fucose; the identity and exact positions of the glycosyl moieties in the T. roseum oscillol diglycoside are yet to be determined. C. Carotenoid modification pathway in T. roseum. Step 1: The introduction of two hydroxyl groups at the ends of lycopene to form (2S, 2′S)-oscillol. The absorbance spectra of T. roseum pigments (Figure 4B) indicate that 1,1′ and 2,2′ positions have all been hydroxylated. We found a 1,1′ hydroxylase in the genome; the gene(s) encoding the 2,2′ hydroxylation function are yet to be identified. Step 2: A glycosyl transferase (CruC) adds a sugar moiety (R) to both ends of (2S,2′S)-oscillol to produce oscillol diglycoside, the dominant carotenoid (Figure 4A, peak 3). The nature of the sugar moieties are unknown. Although the figure shows a 2,2′-oscillol diglycoside, 1,1′-oscillol diglycoside is another possibility at this step. Step 3: The acyltransferase domain of the 1,1′ hydroxylase adds acyl chains (A) to the sugar moieties (R), thus producing the extremely hydrophobic pigments seen in the crude extracts (Figure 4A, peak 1&2).
Figure 5. CO oxidation and CO2 fixation in T. roseum.
A. Two pathways for carbon dioxide fixation and an electronic transport chain for carbon monoxide/hydrogen oxidation in T. roseum. B. Phylogenetic tree for the catalytic domain of Types I-V ribulose bisphosphate carboxylase large subunit RbcL). The tree is based on alignments generated using Pfam domain PF00016. C. Phylogenetic tree for the molybdopterin-binding domain of the aerobic CO dehydrogenase CoxL. The tree is based on alignments generated using Pfam domain PF02738.
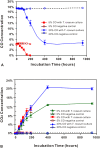
Figure 6. CO consumption and CO2 production by T. roseum cultures.
A. CO concentration in headspace plotted against incubation time. For the 20% CO experiment: (a) O2 concentration (not shown) had decreased to below detection level at 400 hours; and (b) the onset of CO consumption was delayed by roughly 75 hours as compared with the 5% CO experiment. B. CO2 concentration in headspace plotted against incubation time. For the 20% CO experiment, O2 concentration (not shown) had decreased to below detection level at 400 hrs, whereas in the 0% CO control, it fell from an initial level of 20% down to about 0.5%. Initial CO2 production appeared to be highest in the 5% CO experiment (which had an earlier onset of CO oxidation), followed by the 20% CO experiment. Production of CO2 lagged in the T. roseum culture with 0% CO and plateaued at a lower level.
Figure 7. The megaplasmid encoded flagellar system in T. roseum.
A. The three clusters of genes encoding flagellum-related and chemotaxis-related proteins located on the megaplasmid. B. The flagellar structures and related chemotaxis apparatus encoded in the T. roseum genome.
Similar articles
- Thermorudis pharmacophila sp. nov., a novel member of the class Thermomicrobia isolated from geothermal soil, and emended descriptions of Thermomicrobium roseum, Thermomicrobium carboxidum, Thermorudis peleae and Sphaerobacter thermophilus.
Houghton KM, Morgan XC, Lagutin K, MacKenzie AD, Vyssotskii M, Mitchell KA, McDonald IR, Morgan HW, Power JF, Moreau JW, Hanssen E, Stott MB. Houghton KM, et al. Int J Syst Evol Microbiol. 2015 Dec;65(12):4479-4487. doi: 10.1099/ijsem.0.000598. Epub 2015 Sep 14. Int J Syst Evol Microbiol. 2015. PMID: 26374291 - Thermomicrobium carboxidum sp. nov., and Thermorudis peleae gen. nov., sp. nov., carbon monoxide-oxidizing bacteria isolated from geothermally heated biofilms.
King CE, King GM. King CE, et al. Int J Syst Evol Microbiol. 2014 Aug;64(Pt 8):2586-2592. doi: 10.1099/ijs.0.060327-0. Epub 2014 May 9. Int J Syst Evol Microbiol. 2014. PMID: 24814334 - Two Chloroflexi classes independently evolved the ability to persist on atmospheric hydrogen and carbon monoxide.
Islam ZF, Cordero PRF, Feng J, Chen YJ, Bay SK, Jirapanjawat T, Gleadow RM, Carere CR, Stott MB, Chiri E, Greening C. Islam ZF, et al. ISME J. 2019 Jul;13(7):1801-1813. doi: 10.1038/s41396-019-0393-0. Epub 2019 Mar 14. ISME J. 2019. PMID: 30872805 Free PMC article.
Cited by
- Nitrification expanded: discovery, physiology and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi.
Sorokin DY, Lücker S, Vejmelkova D, Kostrikina NA, Kleerebezem R, Rijpstra WI, Damsté JS, Le Paslier D, Muyzer G, Wagner M, van Loosdrecht MC, Daims H. Sorokin DY, et al. ISME J. 2012 Dec;6(12):2245-56. doi: 10.1038/ismej.2012.70. Epub 2012 Jul 5. ISME J. 2012. PMID: 22763649 Free PMC article. - Stress Dependent Biofilm Formation and Bioactive Melanin Pigment Production by a Thermophilic Bacillus Species from Chilean Hot Spring.
Marín-Sanhueza C, Echeverría-Vega A, Gómez A, Cabrera-Barjas G, Romero R, Banerjee A. Marín-Sanhueza C, et al. Polymers (Basel). 2022 Feb 10;14(4):680. doi: 10.3390/polym14040680. Polymers (Basel). 2022. PMID: 35215592 Free PMC article. - Quantification of burkholderia coxL genes in Hawaiian volcanic deposits.
Weber CF, King GM. Weber CF, et al. Appl Environ Microbiol. 2010 Apr;76(7):2212-7. doi: 10.1128/AEM.01861-09. Epub 2010 Feb 5. Appl Environ Microbiol. 2010. PMID: 20139318 Free PMC article. - Trends in Taxonomic and Functional Composition of Soil Microbiome Along a Precipitation Gradient in Israel.
Tripathi BM, Moroenyane I, Sherman C, Lee YK, Adams JM, Steinberger Y. Tripathi BM, et al. Microb Ecol. 2017 Jul;74(1):168-176. doi: 10.1007/s00248-017-0931-0. Epub 2017 Jan 10. Microb Ecol. 2017. PMID: 28074247 - Correlating enzyme annotations with a large set of microbial growth temperatures reveals metabolic adaptations to growth at diverse temperatures.
Engqvist MKM. Engqvist MKM. BMC Microbiol. 2018 Nov 6;18(1):177. doi: 10.1186/s12866-018-1320-7. BMC Microbiol. 2018. PMID: 30400856 Free PMC article.
References
- Fleischmann RD, Adams MD, White O, Clayton RA, Kirkness EF, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269(5223):496–512. - PubMed
- Eisen JA. Assessing evolutionary relationships among microbes from whole-genome analysis. Curr Opin Microbiol. 2000;3(5):475–80. - PubMed
- Jackson TJ, Ramaley RF, Meinschein WG. Thermomicrobium, a new genus of extremely thermophilic bacteria. International Journal of Systematic Bacteriology. 1973;23:28–36.
- Pond JL, Langworthy TA, Holzer G. Long-chain diols: a new class of membrane lipids from a thermophilic bacterium. Science. 1986;231:1134–1136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases