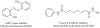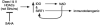The importance of NAD in multiple sclerosis - PubMed (original) (raw)
Review
The importance of NAD in multiple sclerosis
W Todd Penberthy et al. Curr Pharm Des. 2009.
Abstract
The etiology of multiple sclerosis (MS) is unknown but it manifests as a chronic inflammatory demyelinating disease in the central nervous system (CNS). During chronic CNS inflammation, nicotinamide adenine dinucleotide (NAD) concentrations are altered by (T helper) Th1-derived cytokines through the coordinated induction of both indoleamine 2,3-dioxygenase (IDO) and the ADP cyclase CD38 in pathogenic microglia and lymphocytes. While IDO activation may keep auto-reactive T cells in check, hyper-activation of IDO can leave neuronal CNS cells starving for extracellular sources of NAD. Existing data indicate that glia may serve critical functions as an essential supplier of NAD to neurons during times of stress. Administration of pharmacological doses of non-tryptophan NAD precursors ameliorates pathogenesis in animal models of MS. Animal models of MS involve artificially stimulated autoimmune attack of myelin by experimental autoimmune encephalomyelitis (EAE) or by viral-mediated demyelination using Thieler's murine encephalomyelitis virus (TMEV). The Wld(S) mouse dramatically resists razor axotomy mediated axonal degeneration. This resistance is due to increased efficiency of NAD biosynthesis that delays stress-induced depletion of axonal NAD and ATP. Although the Wld(S) genotype protects against EAE pathogenesis, TMEV-mediated pathogenesis is exacerbated. In this review, we contrast the role of NAD in EAE versus TMEV demyelinating pathogenesis to increase our understanding of the pharmacotherapeutic potential of NAD signal transduction pathways. We speculate on the importance of increased SIRT1 activity in both PARP-1 inhibition and the potentially integral role of neuronal CD200 interactions through glial CD200R with induction of IDO in MS pathogenesis. A comprehensive review of immunomodulatory control of NAD biosynthesis and degradation in MS pathogenesis is presented. Distinctive pharmacological approaches designed for NAD-complementation or targeting NAD-centric proteins (SIRT1, SIRT2, PARP-1, GPR109a, and CD38) are outlined towards determining which approach may work best in the context of clinical application.
Figures
Fig. (1)
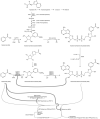
The pathway from NAD precursors to NAD biosynthesis is shown with molecular structures and abbreviations used throughout the manuscript. Also shown, are the reactions that use NAD as a substrate, not a co-factor. Products of ADP cyclase and Sirtuin catalyzed reactions generate products causing release of calcium. CD38 is involved in the persistent release of intracellular calcium connected with hematopoietic chemotaxis and microglial activation, both of which are likely to be of great importance in MS pathogenesis. Feedback inhibition loops are drawn with heavy lines. Additional co-factors required for completion of de novo pathway NAD biosynthesis include ascorbate (vitamin C), pyridoxyl phosphate (vitamin B6), and riboflavin (vitamin B2). Of likely significance to nicotinic acid distinguished capacity as a provider of NAD, NAPRT is not inhibited by NAD feedback inhibition (see text for reference), while NAMPT is inhibited by NAD.
Fig. (2)
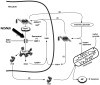
A nuclear SIRT1 mechanism for delaying NAD-ATP depletion through inhibition of PARP-1 is presented. The NAD salvage pathway uses nicotinamide (NAM) as a substrate. NAMPT1 converts nicotinamide (NAM) to nicotinamide mononucleotide (NMN) which via nicotinamide adenylyl-transferase (NMNAT) gets converted to NAD. Both of these processes require ATP. Reactive oxygen/nitrogen species (R(O/N)S) causing DNA damage causes activation of PARP-1, which leads to NAD depletion as NAD is used as a substrate in the polymerization reaction. The salvage pathway is simultaneously activated by (R(O/N)S), which leads to a futile NAD-recycling process that ultimately depletes ATP. Similar lesser characterized ATP depleting pathways are predicted specifically in axonal cytosol and mitochondria where NMNAT2 and NMNAT3 enzymes have been colocalized respectively with Sirtuin and PARP family members. R(O/N)S cause damage to DNA, which activates PARP-1 leading to NAD depletion through use of NAD as a substrate to generate poly(ADP)ribose, PAR (1). Nicotinamide (NAM) is used by nicotinamide phosphoribosyltransferase (NAMPT) to make nicotinamide mononucleotide (NMN). This is then converted to NAD via nicotinamide nucleotide adenylyltransferase 1 (NMNAT1), part of the genomic triplication responsible for the slow Wallerian degeneration mouse (Wlds) phenotype. The NAD salvage pathway repeatedly uses ATP to replenish NAD (2). The NAD-dependent mitochondrial deacetylase, SIRT3, functions as a master regulator of ATP levels. Under ischemic conditions NAD is required to maintain production of ATP from anaerobic glycolysis. The increased salvage pathway efficiency of the Wlds mouse is probably able to provide NAD to maintain ATP generation by anaerobic glycolysis during oxidative stress (3). Otherwise ATP can be supplied via oxidative phosphorylation (4). The futile cycle continues until PAR formation triggers AIF-dependent apoptosis or ATP stores are so depleted that necrosis happens (5). PARP-1 activity can be inhibited directly (e.g. minocycline and PJ-34 are nanomolar affinity inhibitors) or indirectly through activation of SIRT-1 (e.g. resveratrol; 6). Either PARP-1 inhibitory approach can significantly delay NAD and ATP depletion similar to the Wlds mouse. Alternatively pharmacological administration of NAD or precursors can help prevent deficiencies. All of the pharmacological approaches described here have been shown to ameliorate EAE pathogenesis in published reports. Neurons are particularly susceptible to depletion of NAD owing to their apparent lack of a fully functional salvage pathway (see text).
Fig. (3)
The route from dietary NAD precursor to neuron is shown with NAD sinks developing during multiple sclerosis. Astrocytes readily use NAD precursors to generate NAD and can directly transport NAD across the plasma membrane directly via the adenosine receptor P2XY7R (NA, nicotinic acid; NAM, nicotinamide; NAMR, nicotinamide riboside; W, tryptophan; NaMN, nicotinic acid mononucleotide; NMN, nicotinamide mononucleotide; NaAD, nicotinic acid adenine dinucleotide). By contrast neurons are innefficient in this energy dependent process. Similar to several other pathways, it appears as though glia are likely to serve prominent roles in supplying NAD to neurons. With the sudden appearance of lymphocytes in the CNS of the MS patient, exceptional efforts are apparently made to remove lymphocytes by high level IDO induction in microglia and endothelial cells. This decreases the available extracellular tryptophan (W). Additionally, CD38 is highly induced by TNF alpha in professional antigen presenting cells (PAPCs; macrophages, dendritic cells, or microglia) during MS. TNF alpha activation of CD38 leads to degradation of NAD which generates products that stimulate calcium signaling pathways mediating PAPC chemotaxis and activation. PARP-1 activity increases in endothelial cells juxtaposed next to microglial cells in EAE models of MS. The IDO induction in these cells can provide complementary NAD that is lost by CD38 mediated depletion in PAPCs. This persistent inner battle to control the immune system creates a NAD sink that consumes that renders neurons exceptionally vulnerable to stress-induced cell death. Low levels of NAD typically leads to neurodegeneration.
Fig. (4)
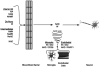
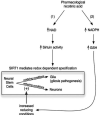
Enzymes controlling NAD metabolism in professional antigen presenting cells (PAPCs; microglia, macrophages, or dendritic cells) are shown with consideration of pharmacological administration of complementary NAD precursors or effectors of NAD utilizing enzymes (SIRT1 activators / PARP-1 inhibitors) towards rescue NAD deficiency arising from chronic inflammatory disease. Immunomodulatory factors exert a coordinated regulation of NAD levels during autoimmune disease or infection. PAPCs including microglia act as sinks acquiring de novo pathway NAD precursor (tryptophan) or degrading NAD directly via activation of IDO and CD38 respectively. CD38 activity is required for chemotaxis (1). Thus, IFNγ activates IDO to increase intracellular NAD while simultaneously activating tryptophanyl-tRNA (encoded by WRS) to maintain essential tryptophan-dependent protein synthesis (2). All three IFNγ-mediated inductions occur in professional antigen presenting cells. IFNγ-mediated activation of IDO leads to complementary increases in NAD levels in a pyridoxyl phosphate (PLP; derived from vitamin B6) co-factor dependent fashion. The anti-epileptic molecule kynurenate is also produced through this pathway. In the end this pathway is predicted to affect global chromatin structure through NAD dependent SIRT-1 and PARP-1 mediated activities as well as other effects through related Sirtuin/PARP family member proteins (3). During chronic inflammation local extracellular NAD sources become deficient (tryptophan and NAD) and this exerts both an anti-proliferative immunotoleragenic effect on T cells but also decreases PAPC chemotaxis while making neighboring cells more vulnerable (4). Accordingly pharmacological application of NAD precursors provides tremendous cytotrophic benefit in numerous models of autoimmune disease. ADPR from either ROS-PARP1-PAR-PARG or excessive CD38 activity can lead to persistent activation of TRPM2 leading to programmed cell death, PCD (5). Excessive CD38 activity has been observed in type 1 diabetics via excessive autoreactive anti-CD38. Highly expressed in the brain and clearly important in immune function, the role of CD38 in MS is completely unexplored. Peroxynitrate can activate PARP1 leading to nuclear PAR formation that translocates to the mitochondria to promote AIF release which also leads to programmed cell death (PCD). Two R(O/N)S sensitive pathways shown at the bottom include DNA damage-PARP1 activation along with direct activation of the redox sensitive TRPM2 divalent cation channel. (6) Administration of pharmacologic doses of NAD precursors (nicotinic acid/niacin, nicotinamide/niacinamide, or nicotinamide riboside) or pharmacologic targeting of NAD-dependent targets (SIRT1 activators or PARP1 inhibitors) may complement the NAD deficiencies arising from immune activation of IDO and CD38.
Fig. (5)
Structures of a new ADP cyclase inhibitor and the first clinical trial inhibitor of constitutive HDACs, not Sirtuins are shown.
Fig. (6)
Inhibition of the constitutive HDACs leads to increased transcriptional expression of SIRT1 and IDO. The increased IDO activity can increase NAD levels which can then further increase SIRT1 activity. Ultimately, SAHA increases immunotolerance. Thus SAHA may have potential in the context of controlling autoreactive pathogenesis in MS.
Fig. (7)
Pharmacological nicotinic acid administration is predicted to favor neuronal genesis over gliogenesis due to the combination of SIRT1 activation combined and increased reducing conditions. SIRT1 controls redox mediated fate specification from neural stem cells. Pharmacological application of nicotinic acid increases NAD which activates SIRT1 (1) while also promoting reducing conditions by increased NADPH and GSH (2). Reducing conditions, being less stressful, allow neuronal specification instead of support cells. Otherwise under oxidizing conditions supporting glial cells are likely to be more immediately needed in order to correct the chemical imbalance, thus potentially favoring pathogenic gliosis. As discussed in section 2.1, minimizing gliosis can help foster remyelination. Thus the dual activity that nicotinic acid exerts by providing both NAD and reducing conditions is expected to respectively increase SIRT1 activity and the neuronal fat specification from stem cells that is likely to be needed for a complete and sustained recovery from MS attacks.
Fig. (8)
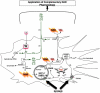
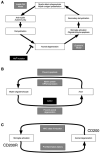
Self-perpetuating neurodegeneration cycles made by interactions between immune cells, microglia, axons and myelin sheaths. a) Anti-myelin immunity cause primary demyelination, leading to axonal degeneration (lesion development from the inside myelin to the outside axon, Outside-In model). Then, degenerated axons activates microglia and induces oligodendrocyte apoptosis, resulting in secondary demyelination (lesion development from the inside axon to the outside myelin, Inside-Out model). Myelin debris would be taken up by microglia and macrophages, which present myelin antigen to autoimmune T cells; this will lead to the second cascade reaction. Wlds mutation prevents axonal degeneration, interfering with a vicious cycle made by the Outside-In and Inside-Out models. b) Axons and myelin require each other in their development and maintenance. Myelin protein abnormality results in axonal degeneration, in which SIRT2 may play a role. Axonal degeneration leads to apoptosis of oligodendrocytes and downregulation of myelin protein mRNA expression. c) Microglia activation and axonal degeneration can make a self-perpetuating cycle. Activated microglia produce proinflammatory factors, and damage axons. Axonal degeneration can activate microglia, including upregulation of MHC class II molecules on the surface of microglia. CD200-CD200R interaction on the neuron-microglia may play a role in the cycle.
Similar articles
- Pharmacological targeting of IDO-mediated tolerance for treating autoimmune disease.
Penberthy WT. Penberthy WT. Curr Drug Metab. 2007 Apr;8(3):245-66. doi: 10.2174/138920007780362545. Curr Drug Metab. 2007. PMID: 17430113 Review. - NAD+ metabolism drives astrocyte proinflammatory reprogramming in central nervous system autoimmunity.
Meyer T, Shimon D, Youssef S, Yankovitz G, Tessler A, Chernobylsky T, Gaoni-Yogev A, Perelroizen R, Budick-Harmelin N, Steinman L, Mayo L. Meyer T, et al. Proc Natl Acad Sci U S A. 2022 Aug 30;119(35):e2211310119. doi: 10.1073/pnas.2211310119. Epub 2022 Aug 22. Proc Natl Acad Sci U S A. 2022. PMID: 35994674 Free PMC article. - Two models for multiple sclerosis: experimental allergic encephalomyelitis and Theiler's murine encephalomyelitis virus.
Tsunoda I, Fujinami RS. Tsunoda I, et al. J Neuropathol Exp Neurol. 1996 Jun;55(6):673-86. doi: 10.1097/00005072-199606000-00001. J Neuropathol Exp Neurol. 1996. PMID: 8642393 Review. - Protective effect and mechanism of nicotinamide adenine dinucleotide against optic neuritis in mice with experimental autoimmune encephalomyelitis.
Guo J, Li B, Wang J, Guo R, Tian Y, Song S, Guo L. Guo J, et al. Int Immunopharmacol. 2021 Sep;98:107846. doi: 10.1016/j.intimp.2021.107846. Epub 2021 Jun 23. Int Immunopharmacol. 2021. PMID: 34174704 - Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.
Rostami A, Ciric B. Rostami A, et al. J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
Cited by
- Berberine: A natural modulator of immune cells in multiple sclerosis.
Yazdanpanah E, Dadfar S, Shadab A, Orooji N, Nemati M, Pazoki A, Esmaeili SA, Baharlou R, Haghmorad D. Yazdanpanah E, et al. Immun Inflamm Dis. 2024 Mar;12(3):e1213. doi: 10.1002/iid3.1213. Immun Inflamm Dis. 2024. PMID: 38477663 Free PMC article. Review. - NAD+ Precursors and Intestinal Inflammation: Therapeutic Insights Involving Gut Microbiota.
Niño-Narvión J, Rojo-López MI, Martinez-Santos P, Rossell J, Ruiz-Alcaraz AJ, Alonso N, Ramos-Molina B, Mauricio D, Julve J. Niño-Narvión J, et al. Nutrients. 2023 Jun 30;15(13):2992. doi: 10.3390/nu15132992. Nutrients. 2023. PMID: 37447318 Free PMC article. Review. - Dynamic changes in kynurenine pathway metabolites in multiple sclerosis: A systematic review.
Fathi M, Vakili K, Yaghoobpoor S, Tavasol A, Jazi K, Mohamadkhani A, Klegeris A, McElhinney A, Mafi Z, Hajiesmaeili M, Sayehmiri F. Fathi M, et al. Front Immunol. 2022 Nov 2;13:1013784. doi: 10.3389/fimmu.2022.1013784. eCollection 2022. Front Immunol. 2022. PMID: 36426364 Free PMC article. - Autoreactive lymphocytes in multiple sclerosis: Pathogenesis and treatment target.
Liu R, Du S, Zhao L, Jain S, Sahay K, Rizvanov A, Lezhnyova V, Khaibullin T, Martynova E, Khaiboullina S, Baranwal M. Liu R, et al. Front Immunol. 2022 Sep 23;13:996469. doi: 10.3389/fimmu.2022.996469. eCollection 2022. Front Immunol. 2022. PMID: 36211343 Free PMC article. Review. - Exploring the roles of tryptophan metabolism in MS beyond neuroinflammation and neurodegeneration: A paradigm shift to neuropsychiatric symptoms.
Tan LSY, Francis HM, Lim CK. Tan LSY, et al. Brain Behav Immun Health. 2021 Jan 4;12:100201. doi: 10.1016/j.bbih.2021.100201. eCollection 2021 Mar. Brain Behav Immun Health. 2021. PMID: 34589733 Free PMC article. Review.
References
- Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: a molecular evaluation of NAD(+) precursor vitamins in human nutrition. Annu Rev Nutr. 2008;28:115–30. - PubMed
- Tang K, Sham H, Hui E, Kirkland JB. Niacin deficiency causes oxidative stress in rat bone marrow cells but not through decreased NADPH or glutathione status. J Nutr Biochem. 2008 Epub ahead of print. - PubMed
- Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci. 2007;32:12–9. - PubMed
- Khan JA, Forouhar F, Tao X, Tong L. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opin Ther Targets. 2007;11:695–705. - PubMed
- Etheridge EW. The butterfly caste: a social history of pellagra in the South. Greenwood Pub. Co; Westport, Conn: 1972.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous