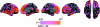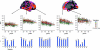High consistency of regional cortical thinning in aging across multiple samples - PubMed (original) (raw)
. 2009 Sep;19(9):2001-12.
doi: 10.1093/cercor/bhn232. Epub 2009 Jan 15.
Lars T Westlye, Inge Amlien, Thomas Espeseth, Ivar Reinvang, Naftali Raz, Ingrid Agartz, David H Salat, Doug N Greve, Bruce Fischl, Anders M Dale, Kristine B Walhovd
Affiliations
- PMID: 19150922
- PMCID: PMC2733683
- DOI: 10.1093/cercor/bhn232
High consistency of regional cortical thinning in aging across multiple samples
Anders M Fjell et al. Cereb Cortex. 2009 Sep.
Abstract
Cross-sectional magnetic resonance imaging (MRI) studies of cortical thickness and volume have shown age effects on large areas, but there are substantial discrepancies across studies regarding the localization and magnitude of effects. These discrepancies hinder understanding of effects of aging on brain morphometry, and limit the potential usefulness of MR in research on healthy and pathological age-related brain changes. The present study was undertaken to overcome this problem by assessing the consistency of age effects on cortical thickness across 6 different samples with a total of 883 participants. A surface-based segmentation procedure (FreeSurfer) was used to calculate cortical thickness continuously across the brain surface. The results showed consistent age effects across samples in the superior, middle, and inferior frontal gyri, superior and middle temporal gyri, precuneus, inferior and superior parietal cortices, fusiform and lingual gyri, and the temporo-parietal junction. The strongest effects were seen in the superior and inferior frontal gyri, as well as superior parts of the temporal lobe. The inferior temporal lobe and anterior cingulate cortices were relatively less affected by age. The results are discussed in relation to leading theories of cognitive aging.
Figures
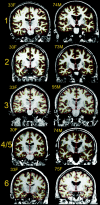
Figure 1.
Example scans from each sample. Scans representative of image quality of 1 young and 1 elderly participant from each of the samples are shown (because sample 4 and 5 are from the same scanner, only examples from sample 4 are shown. All scans are converted from their native format to Freesurfer format. Samples 1, 2, and 4 are taken from Siemens scanners, and 2–4 acquisitions were averaged from each participant to yield high contrast and signal to noise ratio. Sample 2 and 6 are from GE scanners (Signa), with 1 acquisition. The cortex–CSF boundary (red) and the gray–white boundary (yellow) are indicated by the thin line. Anatomical differences between the scans from each sample are incidental.
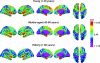
Figure 2.
Mean cortical thickness in 3 age groups. Mean thickness in each hemisphere for the age groups <40 years, 40–60 years, and >60 years are color coded and projected onto an inflated template brain for better visualization of effects buried in sulci. Note that the participants from all the samples are pooled together in each of the age groups, with no corrections for scanner or sample.
Figure 3.
Age effects on cortical thickness in each sample. The figure shows the effect of age on cortical thickness across the entire brain surface when effects of sex were regressed out. The results are color coded and projected onto a semi-inflated template brain for better visualization of effects buried in sulci. Each row represents the results from 1 sample. On the left side of the figure, the effects are thresholded at FDR < 0.05 (corrected for multiple comparisons). On the right side, the results are color coded by use of a wider P value scale.
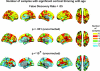
Figure 4.
Consistency across samples. The number of samples in which a statistical effect was reached is color coded and projected onto a semi-inflated template brain. The first row depicts the results when a threshold of FDR < 0.05 was used. As can be seen, age-related thinning of the cerebral cortex is seen in all or 5 of the samples across most of the brain surface. In the second and third row, higher P value thresholds were used.
Figure 5.
Age effects in the total sample. The figure shows the effects of age on cortical thickness when all samples were included in the same analysis (n = 883), with main effect of sample regressed out. In the second row, a higher P value threshold was employed. Even with a P value threshold of 10−25, large effects were seen in several areas.
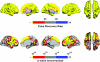
Figure 6.
Sample × age interaction effects. The figure shows which areas of the cerebral cortex that were affected differently by age across samples. The color-coded areas represent significant age × sample interaction effects. Note that the areas which display age × sample interactions are the ones where the strongest age effects were found.
Figure 7.
Scatter plots of mean thickness in selected cortical areas. Manual ROIs were drawn on the inflated template brain surface. The areas in which age and sample interacted were used to guide the manual drawing of ROIs. Mean thickness in different cortical areas were calculated, and plotted against age. The ROIs are shown in the upper row. The scatter plots are shown in the middle row. The participants from each sample are coded in different colors. The last row depicts the Pearson correlation coefficients between age and mean thickness in each of the ROIs. The coefficients are given above each bar if P ≤ 0.05 (uncorrected), and not given if not significant (P > 0.05).
Similar articles
- Development of the Cerebral Cortex across Adolescence: A Multisample Study of Inter-Related Longitudinal Changes in Cortical Volume, Surface Area, and Thickness.
Tamnes CK, Herting MM, Goddings AL, Meuwese R, Blakemore SJ, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, Crone EA, Mills KL. Tamnes CK, et al. J Neurosci. 2017 Mar 22;37(12):3402-3412. doi: 10.1523/JNEUROSCI.3302-16.2017. Epub 2017 Feb 27. J Neurosci. 2017. PMID: 28242797 Free PMC article. - Widespread cortical thinning in children with frontal lobe epilepsy.
Widjaja E, Mahmoodabadi SZ, Snead OC 3rd, Almehdar A, Smith ML. Widjaja E, et al. Epilepsia. 2011 Sep;52(9):1685-91. doi: 10.1111/j.1528-1167.2011.03085.x. Epub 2011 May 31. Epilepsia. 2011. PMID: 21627647 - Association of Epigenetic Metrics of Biological Age With Cortical Thickness.
Proskovec AL, Rezich MT, O'Neill J, Morsey B, Wang T, Ideker T, Swindells S, Fox HS, Wilson TW. Proskovec AL, et al. JAMA Netw Open. 2020 Sep 1;3(9):e2015428. doi: 10.1001/jamanetworkopen.2020.15428. JAMA Netw Open. 2020. PMID: 32926115 Free PMC article. - Differentiating maturational and aging-related changes of the cerebral cortex by use of thickness and signal intensity.
Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Østby Y, Fjell AM. Westlye LT, et al. Neuroimage. 2010 Aug 1;52(1):172-85. doi: 10.1016/j.neuroimage.2010.03.056. Epub 2010 Mar 27. Neuroimage. 2010. PMID: 20347997 - Age-associated alterations in cortical gray and white matter signal intensity and gray to white matter contrast.
Salat DH, Lee SY, van der Kouwe AJ, Greve DN, Fischl B, Rosas HD. Salat DH, et al. Neuroimage. 2009 Oct 15;48(1):21-8. doi: 10.1016/j.neuroimage.2009.06.074. Epub 2009 Jul 4. Neuroimage. 2009. PMID: 19580876 Free PMC article.
Cited by
- Extensive Evaluation of Morphological Statistical Harmonization for Brain Age Prediction.
Lombardi A, Amoroso N, Diacono D, Monaco A, Tangaro S, Bellotti R. Lombardi A, et al. Brain Sci. 2020 Jun 11;10(6):364. doi: 10.3390/brainsci10060364. Brain Sci. 2020. PMID: 32545374 Free PMC article. - Accelerated longitudinal gray/white matter contrast decline in aging in lightly myelinated cortical regions.
Vidal-Piñeiro D, Walhovd KB, Storsve AB, Grydeland H, Rohani DA, Fjell AM. Vidal-Piñeiro D, et al. Hum Brain Mapp. 2016 Oct;37(10):3669-84. doi: 10.1002/hbm.23267. Epub 2016 May 26. Hum Brain Mapp. 2016. PMID: 27228371 Free PMC article. - Large-Scale Morphological Network Efficiency of Human Brain: Cognitive Intelligence and Emotional Intelligence.
Li C, Qiao K, Mu Y, Jiang L. Li C, et al. Front Aging Neurosci. 2021 Feb 24;13:605158. doi: 10.3389/fnagi.2021.605158. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33732136 Free PMC article. - A Novel Brain Network Construction Method for Exploring Age-Related Functional Reorganization.
Li W, Wang M, Li Y, Huang Y, Chen X. Li W, et al. Comput Intell Neurosci. 2016;2016:2429691. doi: 10.1155/2016/2429691. Epub 2016 Feb 29. Comput Intell Neurosci. 2016. PMID: 27057155 Free PMC article. - Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults.
Sakaki M, Yoo HJ, Nga L, Lee TH, Thayer JF, Mather M. Sakaki M, et al. Neuroimage. 2016 Oct 1;139:44-52. doi: 10.1016/j.neuroimage.2016.05.076. Epub 2016 May 31. Neuroimage. 2016. PMID: 27261160 Free PMC article.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Kato N, Ohtomo K. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2008;29:102–116. - PubMed
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. discussion 1279–1282. - PubMed
- Auer S, Reisberg B. The GDS/FAST staging system. Int Psychogeriatr. 1997;9(Suppl. 1):167–171. - PubMed
- Beck AaSR. Beck Depression Inventory scoring manual. New York: The Psychological Corporation; 1987.
- Berg L. Clinical Dementia Rating. Br J Psychiatry. 1984;145:339. - PubMed
Publication types
MeSH terms
Grants and funding
- P41-RR14075/RR/NCRR NIH HHS/United States
- R01-RR13609/RR/NCRR NIH HHS/United States
- R01EB006758/EB/NIBIB NIH HHS/United States
- U54 EB005149/EB/NIBIB NIH HHS/United States
- R01 NS052585-01/NS/NINDS NIH HHS/United States
- R01 EB001550/EB/NIBIB NIH HHS/United States
- R01 AG011230/AG/NIA NIH HHS/United States
- R37 AG011230/AG/NIA NIH HHS/United States
- R01 RR16594-01A1/RR/NCRR NIH HHS/United States
- R01-NS39581/NS/NINDS NIH HHS/United States
- U24 RR021382/RR/NCRR NIH HHS/United States
- R37-AG11230/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical