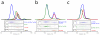Structural characterization of Tip20p and Dsl1p, subunits of the Dsl1p vesicle tethering complex - PubMed (original) (raw)
Structural characterization of Tip20p and Dsl1p, subunits of the Dsl1p vesicle tethering complex
Arati Tripathi et al. Nat Struct Mol Biol. 2009 Feb.
Abstract
Multisubunit tethering complexes are essential for intracellular trafficking and have been proposed to mediate the initial interaction between vesicles and the membranes with which they fuse. Here we report initial structural characterization of the Dsl1p complex, whose three subunits are essential for trafficking from the Golgi apparatus to the endoplasmic reticulum (ER). Crystal structures reveal that two of the three subunits, Tip20p and Dsl1p, resemble known subunits of the exocyst complex, establishing a structural connection among several multisubunit tethering complexes and implying that many of their subunits are derived from a common progenitor. We show, moreover, that Tip20p and Dsl1p interact directly via N-terminal alpha-helices. Finally, we establish that different Dsl1p complex subunits bind independently to different ER SNARE proteins. Our results map out two alternative protein-interaction networks capable of tethering COPI-coated vesicles, via the Dsl1p complex, to ER membranes.
Figures
Figure 1
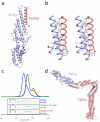
X-ray crystal structures of S. cerevisiae Dsl1p complex subunits. (a) Full-length Tip20p (residues 1-701), color-coded by domain. Two views are shown; it can be seen most clearly in the right panel that the N-terminal helix projects away from the remainder of the protein in a manner stabilized by crystal contacts. (b) Structural alignment of Tip20p and Dsl1ΔC to known exocyst subunits. Shown are S. cerevisiae Sec6p (PDB ID 2FJI, residues 411-805 out of 805), Drosophila melanogaster Sec15 (2A2F, residues 382-699 out of 766), S. cerevisiae Exo70p (2PFV, residues 67-623 out of 623)-, and S. cerevisiae Exo84p (2D2S, residues 525-753 out of 753). Pairwise alignment was performed with the program DaliLite to match each of the exocyst structures to domains C through E of Tip20p; Dsl1ΔC was then aligned to domains A and B of Exo70p. The DaliLite Z scores for the alignments shown were 11.5 (Tip20p-Exo70p), 9.0 (Tip20p-Exo84p), 16.0 (Tip20p-Sec6p), 12.4 (Tip20p-Sec15), and 7.1 (Dsl1ΔC-Exo70p). (c) Dsl1ΔC (residues 37-355 out of 754), color-coded by domain.
Figure 2
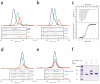
The Tip20p and Dsl1p subunits of the Dsl1p complex form stoichiometric heterodimers. (a) Tip20p binds full-length Dsl1p (residues 1-754). Tip20p alone, Dsl1p alone, or an equimolar mixture were sized on a Superdex 200 gel filtration column. Protein-containing fractions were analyzed using SDS-PAGE gels stained with Coomassie Blue, false-colored to match the corresponding gel filtration profiles. (b) Tip20p binds Dsl1ΔC (residues 1-361). A slight molar excess of Tip20p was present in the mixture (blue gel filtration profile) and accounts for the apparent trailing of the peak. (c) As judged by isothermal titration calorimetry, Tip20p binds Dsl1ΔC with a dissociation constant of 100 nM to form 1:1 complexes. (d) Tip20ΔN (residues 82-701) does not bind Dsl1ΔC, demonstrating that the N-terminal region of Tip20p is essential for heterodimer formation. (e) Tip20p does not bind Dsl1ΔN (residues 57-754), demonstrating that the N-terminal region of Dsl1p is essential for heterodimer formation. (f) The N-terminus of Tip20p (residues 1-43), fused to GST, is sufficient to bind Dsl1ΔC (residues 1-361).
Figure 3
Structural and biochemical characterization of the Tip20p-Dsl1p interaction. (a) X-ray crystal crystal structure of the Tip20p-Dsl1ΔC fusion protein (see text for details). (b) The antiparallel interaction between N-terminal helices of Tip20p and Dsl1p. Side chains are shown for residues in the Tip20p-Dsl1p interface. The side chains of the residues selected for site-directed mutagenesis are labeled and shown as spheres. ‘Intermolecular’ polar interactions are highlighted with black dashed lines. (c) Representative results of Tip20p-Dsl1ΔC binding experiments. Dsl1ΔC binds wild-type Tip20p (top panel; see also Fig. 2b) but not the mutant proteins Tip20p (I10D, L28E) or Tip20p (V17E). A slight molar excess of Dsl1ΔC accounts for the trailing of the blue gel filtration profile. (d) Model for Tip20p-Dsl1ΔC complex generated by replacing Tip20p residues 9-32 in the Tip20p-Dsl1ΔC fusion protein with full-length Tip20p. The model contains a single steric clash, just to the left of the blue “N”, involving a presumably flexible region of Tip20p (see text for details).
Figure 4
Reconstitution of the heterotrimeric Dsl1p complex. (a) Full-length Tip20p, Dsl1p, and Sec39p (residues 1-709) form stoichiometric heterotrimers. (b) Tip20ΔN (residues 82-701) does not bind to Dsl1p-Sec39p heterodimers, demonstrating that the N-terminal region of Tip20p is essential for its incorporation into the Dsl1p complex. (c) Tip20p-Dsl1ΔC heterodimers do not bind Sec39p, demonstrating that a C-terminal region of Dsl1p is essential for incorporation of Sec39p into the Dsl1p complex.
Figure 5
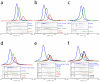
ER SNAREs Sec20p and Use1p bind Dsl1p complex via different subunits. (a) Sec20ΔC (cytoplasmic domain, residues 1-275) binds directly to Tip20p. (b) Sec20ΔC binds the intact Dsl1p complex to form stoichiometric heterotetramers. (c) Use1ΔC (cytoplasmic domain; see text for details) binds directly to Sec39p. (d) Use1ΔC, Sec39p, Dsl1p, and Tip20p form a heterotetrameric complex. (e) Use1ΔC, Sec39p, Dsl1p, Tip20p, and Sec20ΔC form a heteropentameric complex. (f) Use1ΔC-Sec39p-Dsl1p does not bind Tip20ΔN-Sec20ΔC.
Figure 6
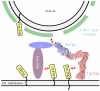
Schematic model for the tethering of Golgi-derived retrograde trafficking vesicles to the ER via bivalent attachment of the Dsl1p complex to the ER SNAREs Use1p and Sec20p. Also shown are two additional SNAREs, Ufe1p and Sec22p, that together with Use1p and Sec20p are thought to form the quaternary SNARE complex that mediates membrane fusion. A central, potentially disorderd region of Dsl1p (residues 388-467) contains binding sites for COPI coat proteins.
Comment in
- Tip20p reaches out to Dsl1p to tether membranes.
Munson M. Munson M. Nat Struct Mol Biol. 2009 Feb;16(2):100-2. doi: 10.1038/nsmb0209-100. Nat Struct Mol Biol. 2009. PMID: 19190660 Free PMC article.
Similar articles
- Golgi-to-endoplasmic reticulum (ER) retrograde traffic in yeast requires Dsl1p, a component of the ER target site that interacts with a COPI coat subunit.
Reilly BA, Kraynack BA, VanRheenen SM, Waters MG. Reilly BA, et al. Mol Biol Cell. 2001 Dec;12(12):3783-96. doi: 10.1091/mbc.12.12.3783. Mol Biol Cell. 2001. PMID: 11739780 Free PMC article. - The Dsl1 tethering complex actively participates in soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptor (SNARE) complex assembly at the endoplasmic reticulum in Saccharomyces cerevisiae.
Diefenbacher M, Thorsteinsdottir H, Spang A. Diefenbacher M, et al. J Biol Chem. 2011 Jul 15;286(28):25027-38. doi: 10.1074/jbc.M110.215657. Epub 2011 Apr 11. J Biol Chem. 2011. PMID: 21482823 Free PMC article. - Tip20p reaches out to Dsl1p to tether membranes.
Munson M. Munson M. Nat Struct Mol Biol. 2009 Feb;16(2):100-2. doi: 10.1038/nsmb0209-100. Nat Struct Mol Biol. 2009. PMID: 19190660 Free PMC article. - Dsl1p/Zw10: common mechanisms behind tethering vesicles and microtubules.
Schmitt HD. Schmitt HD. Trends Cell Biol. 2010 May;20(5):257-68. doi: 10.1016/j.tcb.2010.02.001. Epub 2010 Mar 11. Trends Cell Biol. 2010. PMID: 20226673 Review. - Stx5-Mediated ER-Golgi Transport in Mammals and Yeast.
Linders PT, Horst CV, Beest MT, van den Bogaart G. Linders PT, et al. Cells. 2019 Jul 26;8(8):780. doi: 10.3390/cells8080780. Cells. 2019. PMID: 31357511 Free PMC article. Review.
Cited by
- Retrograde traffic from the Golgi to the endoplasmic reticulum.
Spang A. Spang A. Cold Spring Harb Perspect Biol. 2013 Jun 1;5(6):a013391. doi: 10.1101/cshperspect.a013391. Cold Spring Harb Perspect Biol. 2013. PMID: 23732476 Free PMC article. Review. - ArfGAP1 function in COPI mediated membrane traffic: currently debated models and comparison to other coat-binding ArfGAPs.
Shiba Y, Randazzo PA. Shiba Y, et al. Histol Histopathol. 2012 Sep;27(9):1143-53. doi: 10.14670/HH-27.1143. Histol Histopathol. 2012. PMID: 22806901 Free PMC article. Review. - Quantitative analysis of the native presynaptic cytomatrix by cryoelectron tomography.
Fernández-Busnadiego R, Zuber B, Maurer UE, Cyrklaff M, Baumeister W, Lucic V. Fernández-Busnadiego R, et al. J Cell Biol. 2010 Jan 11;188(1):145-56. doi: 10.1083/jcb.200908082. J Cell Biol. 2010. PMID: 20065095 Free PMC article. - Homology and Modular Evolution of CATCHR at the Origin of the Eukaryotic Endomembrane System.
Santana-Molina C, Gutierrez F, Devos DP. Santana-Molina C, et al. Genome Biol Evol. 2021 Jul 6;13(7):evab125. doi: 10.1093/gbe/evab125. Genome Biol Evol. 2021. PMID: 34061181 Free PMC article. - Molecular architecture of the complete COG tethering complex.
Ha JY, Chou HT, Ungar D, Yip CK, Walz T, Hughson FM. Ha JY, et al. Nat Struct Mol Biol. 2016 Aug;23(8):758-60. doi: 10.1038/nsmb.3263. Epub 2016 Jul 18. Nat Struct Mol Biol. 2016. PMID: 27428773 Free PMC article.
References
- Pfeffer SR. Unsolved mysteries in membrane traffic. Annu. Rev. Biochem. 2007;76:629–645. - PubMed
- Pfeffer SR. Transport-vesicle targeting: tethers before SNAREs. Nat. Cell Biol. 1999;1:E17–E22. - PubMed
- Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev. Cell. 2007;12:671–682. - PubMed
- Gillingham AK, Munro S. Long coiled-coil proteins and membrane traffic. Biochim. Biophys. Acta. 2003;1641:71–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 EB009998/EB/NIBIB NIH HHS/United States
- R01 GM071574/GM/NIGMS NIH HHS/United States
- R01 GM071574-05/GM/NIGMS NIH HHS/United States
- GM071574/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials