Pore dilation occurs in TRPA1 but not in TRPM8 channels - PubMed (original) (raw)
Pore dilation occurs in TRPA1 but not in TRPM8 channels
Jun Chen et al. Mol Pain. 2009.
Abstract
Abundantly expressed in pain-sensing neurons, TRPV1, TRPA1 and TRPM8 are major cellular sensors of thermal, chemical and mechanical stimuli. The function of these ion channels has been attributed to their selective permeation of small cations (e.g., Ca2+, Na+ and K+), and the ion selectivity has been assumed to be an invariant fingerprint to a given channel. However, for TRPV1, the notion of invariant ion selectivity has been revised recently. When activated, TRPV1 undergoes time and agonist-dependent pore dilation, allowing permeation of large organic cations such as Yo-Pro and NMDG+. The pore dilation is of physiological importance, and has been exploited to specifically silence TRPV1-positive sensory neurons. It is unknown whether TRPA1 and TRPM8 undergo pore dilation. Here we show that TRPA1 activation by reactive or non-reactive agonists induces Yo-Pro uptake, which can be blocked by TRPA1 antagonists. In outside-out patch recordings using NMDG+ as the sole external cation and Na+ as the internal cation, TRPA1 activation results in dynamic changes in permeability to NMDG+. In contrast, TRPM8 activation does not produce either Yo-Pro uptake or significant change in ion selectivity. Hence, pore dilation occurs in TRPA1, but not in TRPM8 channels.
Figures
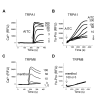
Figure 1
The activation of TRPA1, but not TRPM8, induced Yo-Pro uptake. A, in HEK-293F cells expressing rat TRPA1, AITC elevated intracellular Ca2+, as represented by increases of fluorescence signals (RFU) in the FLIPR based Ca2+ assay. B, in cells expressing TRPA1, AITC evoked robust Yo-Pro uptake in a concentration-dependent manner from the FLIPR based Yo-Pro uptake assays. C, in cells expressing human TRPM8, menthol activated TRPM8 and elevated intracellular Ca2+. D, in cells expressing TRPM8, menthol failed to induce Yo-Pro uptake. Compounds are in μM and additions are indicated by arrows.
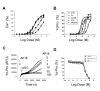
Figure 2
Yo-Pro uptake was evoked by various TRPA1 agonists and blocked by TRPA1 antagonists. Concentration-effect relationships for agonist responses in the Ca2+ assay (A) and Yo-Pro uptake (B). Reactive agonists: AITC, CA and 4-HNE. Non-reactive agonists: FTS and URB597. Data are represented as percentage of maximal AITC responses. C, representative traces of Yo-Pro uptake in response to a first addition of AP18 (0 to 100 μM) and a second addition of AITC (30 μM). AP18 inhibited AITC evoked Yo-Pro uptake in a concentration-dependent manner. D, concentration-effect relationship of Yo-Pro uptake inhibition by AP18, HC-030031 and RR. (n = 4 – 8).
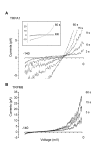
Figure 3
Ionic currents of TRPA1, but not TRPM8, exhibited shifts in reversal potential. Outside-out patches were formed from HeLa cells expressing rat TRPA1 or human TRPM8 plus GFP. NMDG+ was the sole external cation and Na+ was the major internal cation. Membrane potential was held at -80 mV, and a voltage ramp from -140 to 0 mV (500 ms duration) was applied every 3 s immediately. A, current traces from a representative TRPA1-containing patch during 60 s application of AITC (100 μM). To illustrate shifts in Erev, only currents between -6 to 8 pA were plotted. Inset shows the AITC-evoked currents were almost completely blocked by 10 μM RR. B, currents from a representative TRPM8-containing patch during 60 s application of menthol (500 μM). Note the shifts in Erev for TRPA1, but no shift for TRPM8.
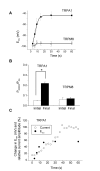
Figure 4
Time-dependent changes in ion permeability occurred in TRPA1 but not in TRPM8. A, Erev values were determined (from Fig. 3 experiments) and plotted as a function of time after application of AITC or menthol. B, permeability ratios (PNMDG/PNa) before and 60 s after agonist addition. Student's t-test was used with p < 0.05 as the criterion for significance (indicated by *). C, changes in Erev and relative currents at 0 mV (from representative recordings) were plotted as a function of time after application of AITC. Outward currents were measured at 0 mV and normalized against the current obtained after 60 s addition of AITC.
Similar articles
- Dynamic changes in the TRPA1 selectivity filter lead to progressive but reversible pore dilation.
Banke TG, Chaplan SR, Wickenden AD. Banke TG, et al. Am J Physiol Cell Physiol. 2010 Jun;298(6):C1457-68. doi: 10.1152/ajpcell.00489.2009. Epub 2010 Mar 24. Am J Physiol Cell Physiol. 2010. PMID: 20457836 - Activation of TRPA1 by membrane permeable local anesthetics.
Leffler A, Lattrell A, Kronewald S, Niedermirtl F, Nau C. Leffler A, et al. Mol Pain. 2011 Aug 23;7:62. doi: 10.1186/1744-8069-7-62. Mol Pain. 2011. PMID: 21861907 Free PMC article. - Activation of TRPA1 channels by the fatty acid amide hydrolase inhibitor 3'-carbamoylbiphenyl-3-yl cyclohexylcarbamate (URB597).
Niforatos W, Zhang XF, Lake MR, Walter KA, Neelands T, Holzman TF, Scott VE, Faltynek CR, Moreland RB, Chen J. Niforatos W, et al. Mol Pharmacol. 2007 May;71(5):1209-16. doi: 10.1124/mol.106.033621. Epub 2007 Feb 21. Mol Pharmacol. 2007. PMID: 17314320 - TRP channels: targets for the relief of pain.
Levine JD, Alessandri-Haber N. Levine JD, et al. Biochim Biophys Acta. 2007 Aug;1772(8):989-1003. doi: 10.1016/j.bbadis.2007.01.008. Epub 2007 Jan 23. Biochim Biophys Acta. 2007. PMID: 17321113 Review. - TRPA1.
García-Añoveros J, Nagata K. García-Añoveros J, et al. Handb Exp Pharmacol. 2007;(179):347-62. doi: 10.1007/978-3-540-34891-7_21. Handb Exp Pharmacol. 2007. PMID: 17217068 Review.
Cited by
- Characterization of a ligand binding site in the human transient receptor potential ankyrin 1 pore.
Klement G, Eisele L, Malinowsky D, Nolting A, Svensson M, Terp G, Weigelt D, Dabrowski M. Klement G, et al. Biophys J. 2013 Feb 19;104(4):798-806. doi: 10.1016/j.bpj.2013.01.008. Biophys J. 2013. PMID: 23442958 Free PMC article. - Regulation of vascular tone by transient receptor potential ankyrin 1 channels.
Thakore P, Ali S, Earley S. Thakore P, et al. Curr Top Membr. 2020;85:119-150. doi: 10.1016/bs.ctm.2020.01.009. Epub 2020 Feb 29. Curr Top Membr. 2020. PMID: 32402637 Free PMC article. Review. - TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity.
Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, Blackshaw LA. Brierley SM, et al. J Physiol. 2011 Jul 15;589(Pt 14):3575-93. doi: 10.1113/jphysiol.2011.206789. Epub 2011 May 9. J Physiol. 2011. PMID: 21558163 Free PMC article. - The transient receptor potential channel TRPA1: from gene to pathophysiology.
Nilius B, Appendino G, Owsianik G. Nilius B, et al. Pflugers Arch. 2012 Nov;464(5):425-58. doi: 10.1007/s00424-012-1158-z. Epub 2012 Sep 22. Pflugers Arch. 2012. PMID: 23001121 Review. - Effect of cholesterol depletion on the pore dilation of TRPV1.
Jansson ET, Trkulja CL, Ahemaiti A, Millingen M, Jeffries GD, Jardemark K, Orwar O. Jansson ET, et al. Mol Pain. 2013 Jan 2;9:1. doi: 10.1186/1744-8069-9-1. Mol Pain. 2013. PMID: 23279936 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous