Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner - PubMed (original) (raw)
Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner
Colin K Choi et al. Nat Cell Biol. 2008 Sep.
Abstract
Using two-colour imaging and high resolution TIRF microscopy, we investigated the assembly and maturation of nascent adhesions in migrating cells. We show that nascent adhesions assemble and are stable within the lamellipodium. The assembly is independent of myosin II but its rate is proportional to the protrusion rate and requires actin polymerization. At the lamellipodium back, the nascent adhesions either disassemble or mature through growth and elongation. Maturation occurs along an alpha-actinin-actin template that elongates centripetally from nascent adhesions. Alpha-Actinin mediates the formation of the template and organization of adhesions associated with actin filaments, suggesting that actin crosslinking has a major role in this process. Adhesion maturation also requires myosin II. Rescue of a myosin IIA knockdown with an actin-bound but motor-inhibited mutant of myosin IIA shows that the actin crosslinking function of myosin II mediates initial adhesion maturation. From these studies, we have developed a model for adhesion assembly that clarifies the relative contributions of myosin II and actin polymerization and organization.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
Figure 1
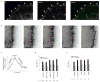
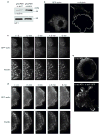
Rapid turnover of nascent adhesions during protrusion. (a) Expressed human β1 integrin (green) and endogenous paxillin (magenta) imaged using TIRF optics in CHO.K1 cells. Both localize in nascent adhesions that form a thin outline near the leading edge (arrowheads). (b) The rapid assembly (green arrowheads) of new nascent adhesions near the leading edge as the pre-existing adhesions disassemble behind them (pink arrowheads), observed from TIRF time-lapse images of paxillin–GFP in CHO.K1 cells. These panels correspond to Supplementary Information, Movie 1. (c) Temporal fluorescence intensity profile of paxillin–GFP in a representative nascent adhesion. The lifetime of the nascent adhesion can be resolved into three discrete phases: assembly, stability and disassembly. (d) Rate constants for nascent adhesion assembly and disassembly. Nascent adhesions assemble and turnover at comparable rates independently of fibronectin (FN) concentration adsorbed at a concentration of 2 μg ml−1 and 20 μg ml−1) and protrusive phenotype (MIIA knockdown, expression of S273D–paxillin, expression of CA-PAK). Data are mean ± s.e.m., measured from 10–20 individual adhesions in 4–8 cells from independent experiments. (e) Rate constants for the assembly and disassembly of adhesion molecules in nascent adhesions. All molecules entered and exited the nascent adhesions simultaneously and at comparable rates. Data are mean ± s.e.m., measured from 10–15 individual adhesions in 4–6 cells from independent experiments. Scale bars are 10 μm (a) and 3 μm (b).
Figure 2
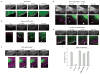
Myosin II inhibition promotes nascent adhesion assembly and inhibits adhesion maturation. (a) CHO.K1 cells were transfected with pSUPER vector alone (control) or pSUPER-MIIA and pSUPER-MIIB. The cells were simultaneously stained for MIIA and MIIB. The reduced expression is evident in the transfected cell, which is silhouetted to show its outline. (b) Time-lapse TIRF images of a MIIA/MIIB-depleted cell expressing paxillin–mOrange. Images are representative of more than 10 cells from four independent experiments. (c) Time-lapse TIRF images of paxillin–mOrange in a MIIA-depleted cell treated with blebbistatin (20 μM, Blebb). Note the rim of nascent adhesions at the periphery. Scale bars are 10 μm (a) and 5 μm (b, c).
Figure 3
Nascent adhesions reside in the lamellipodium. (a) Time-lapse TIRF images of GFP–actin (green) and paxillin–mOrange (magenta) show nascent adhesions forming and residing exclusively in the lamellipodium. These panels correspond to Supplementary Information, Movie 2. (b) Dual-colour temporal profiles of GFP–actin of the lamellipodium and paxillin–mOrange in a representative nascent adhesion. Relative changes in fluorescent intensities show that nascent adhesions begin to assemble after actin appears in the position of the adhesions (that is, assembly occurs within the protruding lamellipodium). Next, the stability phase correlates with residence time of the adhesion in the lamellipodium. Finally, the intensity of paxillin decreases simultaneously with that of actin, indicating that nascent adhesions turnover when the lamellipodium (dense actin band) moves by the adhesions. (c) Comparison of protrusion rate with assembly and disassembly rates of nascent adhesions from cells on fibronectin (2 μg ml−1). Each data point represents the kinetics of one adhesion and its adjacent cell periphery, which were quantified by measuring temporal fluorescent intensities of paxillin–GFP (nascent adhesion) and cytoplasmic mCherry (protrusion). The analyses were performed during the time of nascent adhesion assembly/disassembly. Regression line shows a linear correlation between the rates of assembly and protrusion. Rates were measured from 30 individual adhesions and protrusions in 11 cells from independent experiments. (d) Application of cytochalasin-D (1 μM) halts the protrusion and constricts the lamellipodium immediately (30 s). Minutes later (5 min), the thick band of GFP–actin characterizing the lamellipodium collapses, and the nascent adhesions residing in the band, indicated by paxillin–mOrange, disassemble. Scale bars are 3 μm (a, d)
Figure 4
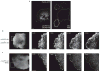
An actin template organizes and promotes hierarchical adhesion maturation. (a) TIRF images of paxillin–GFP and GFP–actin exhibiting centripetal elongation when protrusion halts (green arrowheads). The linear structures emerged from the cell edge. These panels correspond to Supplementary Information, Movies 3 and 5. (b) Time-lapse TIRF images of GFP–actin (green) and paxillin–mKO (magenta) show elongating actin templates and elongating adhesions, respectively. During protrusion, nascent adhesions reside in the lamellipodium. Once the leading edge halts, a population of the adhesions matures along the growing actin filaments (arrowheads). These panels correspond to Supplementary Information, Movie 6. (c) Time-lapse TIRF images of actin and α-actinin shows simultaneous elongations from the cell periphery. Vinculin, talin and paxillin incorporation was delayed, compared with α-actinin. Inset bars indicate the degree of elongation (green, top panels; magenta, bottom panels). Scale bars are 5 μm (a) and 3 μm (b, c).
Figure 5
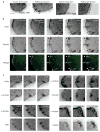
α-actinin knockdown inhibits actin orientation and adhesion elongation in protrusions. (a) Immunoblot of α-actinin in CHO.K1 cells transfected with pSUPER–GFP vector (control) or pSUPER–GFP-RNAi against α-actinin (α-act1). The GIT1 immunoblot was used as a loading control. (b) Representative images of α-actinin-depleted cells stained for α-actinin. The transfected cell is silhouetted. (c, d) Time-lapse TIRF images of control (c) or α-actinin-depleted (d) cells expressing GFP–actin (top) or paxillin–mOrange (bottom). Panels and movies are representative of more than 25 cells in 6 independent experiments. Note the short, mis-oriented actin filaments and dot-like adhesions in α-actinin-depleted cells. Knockdown panels correspond to Supplementary Information, Movie 8. (e) TIRF images of α-actinin-depleted cells (α-act KD, top) and α-actinin-depleted cells rescued with an RNAi-insensitive α-actinin (α-act KD + α-actR–GFP, bottom) expressing paxillin–mOrange. Note the elongated adhesions in the rescued cells. Scale bars are 10 μm (b, e) and 5 μm (c, d).
Figure 6
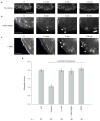
N93K–myosin IIA or overexpressed α-actinin restores adhesion maturation in myosin IIA-deficient cells. (a–e) Time-lapse TIRF images of MIIA-depleted CHO.K1 cells expressing GFP–actin (control, a), GFP–MIIA (rescue, b), GFP–MIIA–N93K (c), GFP–MIIA–N93K with blebbistatin (20 μM, d), and α-actinin–GFP (e). Black and white panels show adhesions as revealed by co-expression of paxillin–mOrange in each case. Colour inserts are magnifications of the indicated regions in the black and white panels. Paxillin–mOrange is depicted in magenta in all cases and green represents GFP–actin (a), GFP–MIIA (b), GFP–MIIA-N93K (c, d) or α-actinin–GFP (e). Arrowheads indicate representative maturing adhesions. Panels a–c correspond to Supplementary Information, Movie 9 and panels d to Supplementary Information, Movie 10. (f) Quantification of the elongation index of maturing adhesions under the different conditions. Data are mean ± s.e.m., measured from more than 25 adhesions from 5–6 cells per condition (n = 30 for each condition). N/A, not applicable (MIIA-deficient cells contain no elongating adhesions). Scale bars are 5 μm.
Figure 7
Rescue of adhesion maturation in α-actinin-depleted cells by overexpression of MIIA. (a–c) Time-lapse TIRF images of α-actinin-depleted CHO.K1 cells expressing paxillin–mOrange and GFP–actin (a), GFP–MIIA–N93K (b) and GFP–MIIA (c). For convenience, only paxillin is shown. Arrowheads indicate representative maturing adhesions. Scale bars are 5 μm. These panels correspond to Supplementary Information, Movie 11. (d) Quantification of the elongation index of maturing adhesions in the different conditions. Data are mean ± s.e.m., measured from more than 25 adhesions from 5–6 cells per condition; *P = 6 × 10−9, Student’s two-tailed _t_-test.
Figure 8
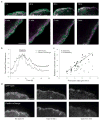
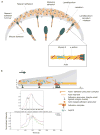
Working model for adhesion assembly, turnover and maturation (a) During protrusion, adhesions initially assemble as punta (blue circle) in the lamellipodium (gray band); their formation is driven by or linked to actin polymerization. Following assembly, these nascent adhesions remain small and stable within the lamellipodium. The nascent adhesions turnover (clear circle) when the depolymerizing dendritic at the rear of the lamellipodium passes by them; this links the stability of these adhesions to the integrity of the dendritic actin. The formation and turnover of nascent adhesions do not require myosin II activity. In addition, nascent adhesions can grow along an actin template (maturing adhesions), which elongates centripetally at the lamellipodium-lamellum interface. The cross-linking activities of both myosin II and α-actinin, possibly in conjunction with contraction, are critical for the initial elongation of adhesions, and α-actinin is also required for the proper positioning of adhesions on actin filaments. Working synergistically with contraction, the cross-linking of actin by myosin II and α-actinin mediate further development and maturation of the adhesions.(b) Quantitative analysis of the mathematical model of actin and adhesion assembly in the lamellipodium predicts accurately the dynamic and exclusive nature of nascent adhesions in the lamellipodium in migrating cells. Top plot, mathematical model; Bottom plot, representative experimental result. V = protrusion rate; L = width of the actin branching zone; X = distance from the front to the rear; τ = time lag. See the Supplementary Information, Materials section for a detailed description of the model, including its assumptions, governing equations, and solutions.
Similar articles
- Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells.
Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Vicente-Manzanares M, et al. J Cell Biol. 2007 Feb 26;176(5):573-80. doi: 10.1083/jcb.200612043. Epub 2007 Feb 20. J Cell Biol. 2007. PMID: 17312025 Free PMC article. - STED analysis reveals the organization of nonmuscle muscle II, muscle myosin II, and F-actin in nascent myofibrils.
Wang J, Fan Y, Sanger JM, Sanger JW. Wang J, et al. Cytoskeleton (Hoboken). 2022 Dec;79(12):122-132. doi: 10.1002/cm.21729. Epub 2022 Sep 30. Cytoskeleton (Hoboken). 2022. PMID: 36125330 - Myosin IIA/IIB restrict adhesive and protrusive signaling to generate front-back polarity in migrating cells.
Vicente-Manzanares M, Newell-Litwa K, Bachir AI, Whitmore LA, Horwitz AR. Vicente-Manzanares M, et al. J Cell Biol. 2011 Apr 18;193(2):381-96. doi: 10.1083/jcb.201012159. Epub 2011 Apr 11. J Cell Biol. 2011. PMID: 21482721 Free PMC article. - Regulation of actin assembly associated with protrusion and adhesion in cell migration.
Le Clainche C, Carlier MF. Le Clainche C, et al. Physiol Rev. 2008 Apr;88(2):489-513. doi: 10.1152/physrev.00021.2007. Physiol Rev. 2008. PMID: 18391171 Review. - Assembly and mechanosensory function of focal adhesions: experiments and models.
Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM. Bershadsky AD, et al. Eur J Cell Biol. 2006 Apr;85(3-4):165-73. doi: 10.1016/j.ejcb.2005.11.001. Epub 2005 Dec 19. Eur J Cell Biol. 2006. PMID: 16360240 Review.
Cited by
- Vinculin-actin interaction couples actin retrograde flow to focal adhesions, but is dispensable for focal adhesion growth.
Thievessen I, Thompson PM, Berlemont S, Plevock KM, Plotnikov SV, Zemljic-Harpf A, Ross RS, Davidson MW, Danuser G, Campbell SL, Waterman CM. Thievessen I, et al. J Cell Biol. 2013 Jul 8;202(1):163-77. doi: 10.1083/jcb.201303129. J Cell Biol. 2013. PMID: 23836933 Free PMC article. - Optineurin links Hace1-dependent Rac ubiquitylation to integrin-mediated mechanotransduction to control bacterial invasion and cell division.
Petracchini S, Hamaoui D, Doye A, Asnacios A, Fage F, Vitiello E, Balland M, Janel S, Lafont F, Gupta M, Ladoux B, Gilleron J, Maia TM, Impens F, Gagnoux-Palacios L, Daugaard M, Sorensen PH, Lemichez E, Mettouchi A. Petracchini S, et al. Nat Commun. 2022 Oct 13;13(1):6059. doi: 10.1038/s41467-022-33803-x. Nat Commun. 2022. PMID: 36229487 Free PMC article. - From stress fiber to focal adhesion: a role of actin crosslinkers in force transmission.
Katsuta H, Sokabe M, Hirata H. Katsuta H, et al. Front Cell Dev Biol. 2024 Aug 13;12:1444827. doi: 10.3389/fcell.2024.1444827. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39193363 Free PMC article. Review. - Spatiotemporal control of actomyosin contractility by MRCKβ signaling drives phagocytosis.
Zihni C, Georgiadis A, Ramsden CM, Sanchez-Heras E, Haas AJ, Nommiste B, Semenyuk O, Bainbridge JWB, Coffey PJ, Smith AJ, Ali RR, Balda MS, Matter K. Zihni C, et al. J Cell Biol. 2022 Nov 7;221(11):e202012042. doi: 10.1083/jcb.202012042. Epub 2022 Sep 19. J Cell Biol. 2022. PMID: 36121394 Free PMC article. - Smooth muscle hyperplasia due to loss of smooth muscle α-actin is driven by activation of focal adhesion kinase, altered p53 localization and increased levels of platelet-derived growth factor receptor-β.
Papke CL, Cao J, Kwartler CS, Villamizar C, Byanova KL, Lim SM, Sreenivasappa H, Fischer G, Pham J, Rees M, Wang M, Chaponnier C, Gabbiani G, Khakoo AY, Chandra J, Trache A, Zimmer W, Milewicz DM. Papke CL, et al. Hum Mol Genet. 2013 Aug 1;22(15):3123-37. doi: 10.1093/hmg/ddt167. Epub 2013 Apr 15. Hum Mol Genet. 2013. PMID: 23591991 Free PMC article.
References
- Webb DJ, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nature Cell Biol. 2004;6:154–161. - PubMed
- Ridley AJ, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. - PubMed
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. - PubMed
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nature Cell Biol. 2002;4:E65–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM023244/GM/NIGMS NIH HHS/United States
- U54 GM064346/GM/NIGMS NIH HHS/United States
- GM23244/GM/NIGMS NIH HHS/United States
- U54 GM064346-08/GM/NIGMS NIH HHS/United States
- R37 GM023244/GM/NIGMS NIH HHS/United States
- R01 GM023244-33/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources