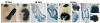Tamoxifen-dependent, inducible Hoxb6CreERT recombinase function in lateral plate and limb mesoderm, CNS isthmic organizer, posterior trunk neural crest, hindgut, and tailbud - PubMed (original) (raw)
Tamoxifen-dependent, inducible Hoxb6CreERT recombinase function in lateral plate and limb mesoderm, CNS isthmic organizer, posterior trunk neural crest, hindgut, and tailbud
Minh-Thanh Nguyen et al. Dev Dyn. 2009 Feb.
Abstract
The ability to generate conditional mutant alleles in mice using Cre-lox technology has facilitated analysis of genes playing critical roles in multiple developmental processes at different times. We used a transgenic Hoxb6 promoter to drive tamoxifen-dependent Cre recombinase expression in several developing systems that serve as major models for elucidating inductive interactions and mechanisms of morphogenesis, including lateral plate mesoderm and descendant limb buds, neural crest progenitors of the neural tube, tailbud, and CNS isthmic organizer. The Hoxb6CreER(T) line gives very rapid and complete recombination over a short time window after a single tamoxifen dose, allowing precise time requirements for gene function to be assessed accurately. Embryonic cells cultured from the Hoxb6CreER(T) line also display rapid recombination ex vivo after tamoxifen exposure. Hence, the Hoxb6CreER(T) line provides a valuable tool for analyzing gene function, as well as lineage tracing studies using genetic cell marking, in several developing systems.
Figures
Figure 1
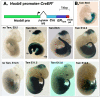
Generation of a Hoxb6CreERT line and activity in early embryos at 24 hrs after administration of a single tamoxifen dose. A. Schematic diagram of transgenic Hoxb6CreERT expression construct used to generate transgenic mice, which includes 3.6kbp of the Hoxb6 promoter with limb-lateral plate enhancer, the β-globin intron (IVS), and the SV40 polyA additional signal. B. Overview of tamoxifen-dependent recombination in CNS, lateral plate and tailbud during early development (E8.0-12.5). A single dose tamoxifen was injected IP at the times indicated in each panel, and embryos were harvested and stained for LacZ activity at 24 hours post-injection. The far left panels (top and bottom) show representative embryos at E9.5 and at E12.5 stained for LacZ reporter activity without any tamoxifen treatment, demonstrating level of background Cre activity. Cre activity in lateral plate and ventral body wall is already evident after tamoxifen treatment at E8, and does not decline appreciably until about E12. Activity in midbrain-hindbrain neuroectoderm including the junctional isthmic organizer (io) and in tailbud (tb) is detected after tamoxifen treatment at E8.5 and remains robust until E11.5-12, as does activity in the forelimb (flb) and hindlimb (hb) buds. Arrow in E12.5 treated panel shows mammary bud staining.
Figure. 2
Time course and recombination efficiency of Hoxb6CreERT in developing limb buds following single dose Tamoxifen injection at E8.5. (A-D) Single dose tamoxifen resulted in strong LacZ reporter activity throughout both lateral plate mesoderm and midbrain-hindbrain junctional region within 12 hrs after injection (A), as well as within very early tailbud, apparent at 18 hrs (B, D). Sectioning at 18 hrs after treatment (levels of sections C, D are indicated in B) showed complete recombination in both somatic/parietal and splanchnic/visceral lateral plate mesoderm, as well as caudal midgut and hindgut (hg), caudal dorsal neural tube and incipient tailbud (C, D; see also Fig. 5E). (E-H) At 24 hrs, when the forelimb bud has emerged, both wholemount embryos (E), and sections (F-H; levels of sections are indicated in E) reveal strong activity extending rostrally up to the anterior 1/4 forelimb bud mesoderm (F,G). (I) The ectoderm, including mature AER shown at E10.5, is completely negative for Hoxb6 promoter expression and Cre recombinase activity. (J) At E13.5, the anterior-most mesoderm of the forelimb remains negative for LacZ reporter activity and this persistently negative region has given rise to digit one (arrow; see also Fig. 4). All panels are oriented with rostral at the top.
Figure 3
Efficiency of Tamoxifen-regulated recombination by Hoxb6CreERT following single Tamoxifen injection at E9.5 or E10.5. A single tamoxifen dose at either E9.5 (A) or at E10.5 (B) gave strong recombination, as judged by LacZ reporter activity, in the posterior 3/4 of forelimb (FL) and throughout hindlimb (HL) buds by 12 hrs after administration. However, for E10.5, reporter recombination in the flank and ventral body wall region and the tailbud required 24 hrs to appear complete. Sections at 12 hrs (A-lower FL and HL panels) or at 18hrs (B- FL and HL panels) showed complete recombination within domains of expression in the limb bud mesoderm. All panels are oriented with rostral at the top.
Figure 4
Extent of recombination assayed in later-stage limb buds and decline of Hoxb6CreERT activity in cells undergoing chondrogenic differentiation. Embryos received a single dose tamoxifen administered at the stages indicated at the top (ranging from E9.5 to E12), and were collected at between 24-48 hrs after treatment (E12-E14) and sectioned to assess recombination in mesodermal tissues. Whole mount panels (upper panels) show examples of forelimbs (FL) and hindlimbs (HL) from embryos collected between E12.5-E13.5. Note that the digit 1 region of the FL is always negative for LacZ activity. The HL LacZ activity is uniform along the limb A-P axis until about E12, when the mid-region shows weaker activity than the anterior and posterior borders (arrows). The sections of limbs shown in lower panels were from embryos collected at E13 (for E9.5 and E10.5 injections), E12 (for E11 injection), E13.5 (for E11.5), and at E14 (for E12 tamoxifen IP injection). As previously reported (Schughart et al, 1991), the Hoxb6 promoter activity driving Cre expression is restricted mainly to undifferentiated mesenchyme, and declines as chondrogenic differentiation proceeds. Reduced LacZ activity is evident in digit condensations compared to interdigital mesenchyme on sections from embryos that were treated with tamoxifen at E11 or later for FL, and E11.5 or later for HL. All panels are oriented with rostral at the top.
Figure 5
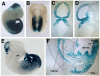
Hoxb6CreERT activity in non-limb sites: CNS isthmic organizer, caudal neural crest, mid/hindgut, and tailbud. (A-D) Strong Cre activity in the midbrain-hindbrain junction was evident on wholemount staining for LacZ reporter activity beginning at E8.5 (panel B, tamoxifen injected at E8.5; collected after 24 hrs and stained for LacZ activity). Embryos exposed to tamoxifen at E10.5 and collected at 24 hrs. (wholemount shown in panel A) were also sectioned through the head and the body to evaluate recombination within non-limb tissues in which the Hoxb6 transgenic promoter is active. Cre activity in the head CNS was highly restricted to the midbrain-hindbrain junctional region (C-frontal section, D-sagital section) and complete recombination was seen within the isthmic organizer zone. (E) Sagitally bisected whole mount embryo collected 24 hrs after tamoxifen treatment at E10.5 revealed gut LacZ staining beginning at the entry of midgut through the umbilical hernia (arrow), whereas the foregut (pharynx, ph; stomach, st) showed no LacZ activity. (F) In sagital histologic sections, the caudal midgut and entire hindgut endoderm (mg, hg), along with immediately subjacent mesenchymal cells, showed complete recombination; similar to earlier stages (see Fig. 2C, D, H). Sagital sections also showed recombination in caudal dorsal root ganglia (DRG), consistent with the earlier observed LacZ activity in the dorsal neural tube (from which neural crest arises) in the caudal embryo (see also Fig. 2D, H; 3A). The tail region (tb) showed expression in tissues of all germ layers including the entire neural tube (nt) and epithelial somites (epi so) descendent from tailbud mesoderm.
Figure 6
Efficiency of Hoxb6CreERT activity ex vivo in cultured limb bud cells. Dissociated cells from E10.25 hindlimb buds of Hoxb6CreERT; RosaLacZ embryos were cultured as described previously (Knezevic et al, 1997). Cultures were exposed to 1 uM 4-OH-tamoxifen or to vehicle alone (control) immediately after plating, harvested at the times indicated and stained for LacZ activity. Untreated control cultures showed a few scattered cells with LacZ activity (~4%). Cultures exposed to 4-OH-tamoxifen showed substantial, but not complete recombination after 12 hrs (~ 50%). By 24 hrs, recombination was essentially complete (~95%) and comparable to 48 hrs. Notably, there were always a few cells (~2%) remaining negative for LacZ activity, possibly reflecting cell types not expressing the Hoxb6 promoter.
Similar articles
- HoxB6-Cre transgenic mice express Cre recombinase in extra-embryonic mesoderm, in lateral plate and limb mesoderm and at the midbrain/hindbrain junction.
Lowe LA, Yamada S, Kuehn MR. Lowe LA, et al. Genesis. 2000 Feb;26(2):118-20. doi: 10.1002/(sici)1526-968x(200002)26:2<118::aid-gene5>3.0.co;2-s. Genesis. 2000. PMID: 10686603 No abstract available. - Fate maps of neural crest and mesoderm in the mammalian eye.
Gage PJ, Rhoades W, Prucka SK, Hjalt T. Gage PJ, et al. Invest Ophthalmol Vis Sci. 2005 Nov;46(11):4200-8. doi: 10.1167/iovs.05-0691. Invest Ophthalmol Vis Sci. 2005. PMID: 16249499 - A Tlx2-Cre mouse line uncovers essential roles for hand1 in extraembryonic and lateral mesoderm.
Maska EL, Cserjesi P, Hua LL, Garstka ME, Brody HM, Morikawa Y. Maska EL, et al. Genesis. 2010 Aug;48(8):479-84. doi: 10.1002/dvg.20644. Genesis. 2010. PMID: 20506548 Free PMC article. - Lineage tracing of axial progenitors using Nkx1-2CreERT2 mice defines their trunk and tail contributions.
Rodrigo Albors A, Halley PA, Storey KG. Rodrigo Albors A, et al. Development. 2018 Oct 2;145(19):dev164319. doi: 10.1242/dev.164319. Development. 2018. PMID: 30201686 Free PMC article. - How the embryo makes a limb: determination, polarity and identity.
Tickle C. Tickle C. J Anat. 2015 Oct;227(4):418-30. doi: 10.1111/joa.12361. Epub 2015 Aug 7. J Anat. 2015. PMID: 26249743 Free PMC article. Review.
Cited by
- Non-canonical Wnt5a/Ror2 signaling regulates kidney morphogenesis by controlling intermediate mesoderm extension.
Yun K, Ajima R, Sharma N, Costantini F, Mackem S, Lewandoski M, Yamaguchi TP, Perantoni AO. Yun K, et al. Hum Mol Genet. 2014 Dec 20;23(25):6807-14. doi: 10.1093/hmg/ddu397. Epub 2014 Jul 31. Hum Mol Genet. 2014. PMID: 25082826 Free PMC article. - A mesodermal fate map for adipose tissue.
Sebo ZL, Jeffery E, Holtrup B, Rodeheffer MS. Sebo ZL, et al. Development. 2018 Aug 17;145(17):dev166801. doi: 10.1242/dev.166801. Development. 2018. PMID: 30045918 Free PMC article. - The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function.
Isern J, García-García A, Martín AM, Arranz L, Martín-Pérez D, Torroja C, Sánchez-Cabo F, Méndez-Ferrer S. Isern J, et al. Elife. 2014 Sep 25;3:e03696. doi: 10.7554/eLife.03696. Elife. 2014. PMID: 25255216 Free PMC article. - Proximo-distal positional information encoded by an Fgf-regulated gradient of homeodomain transcription factors in the vertebrate limb.
Delgado I, López-Delgado AC, Roselló-Díez A, Giovinazzo G, Cadenas V, Fernández-de-Manuel L, Sánchez-Cabo F, Anderson MJ, Lewandoski M, Torres M. Delgado I, et al. Sci Adv. 2020 Jun 3;6(23):eaaz0742. doi: 10.1126/sciadv.aaz0742. eCollection 2020 Jun. Sci Adv. 2020. PMID: 32537491 Free PMC article. - A spatio-temporally constrained gene regulatory network directed by PBX1/2 acquires limb patterning specificity via HAND2.
Losa M, Barozzi I, Osterwalder M, Hermosilla-Aguayo V, Morabito A, Chacón BH, Zarrineh P, Girdziusaite A, Benazet JD, Zhu J, Mackem S, Capellini TD, Dickel D, Bobola N, Zuniga A, Visel A, Zeller R, Selleri L. Losa M, et al. Nat Commun. 2023 Jul 6;14(1):3993. doi: 10.1038/s41467-023-39443-z. Nat Commun. 2023. PMID: 37414772 Free PMC article.
References
- Ahn S, Joyner AL. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell. 2004;118:505–516. - PubMed
- Arques CG, Doohan R, Sharpe J, Torres M. Cell tracing reveals a dorsoventral lineage restriction plane in the mouse limb bud mesenchyme. Development. 2007;134:3713–3722. - PubMed
- Becker D, Jiang Z, Knodler P, Deinard AS, Eid R, Kidd KK, Shashikant CS, Ruddle FH, Schughart K. Conserved regulatory element involved in the early onset of Hoxb6 gene expression. Dev. Dynamics. 1996;205:73–81. - PubMed
- Chevallier A, Kieny M, Mauger A. Limb-somite relationship: origin of the limb musculature. J. Embryol. Exp. Morphol. 1977;13:3231–3243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases