Alternative zippering as an on-off switch for SNARE-mediated fusion - PubMed (original) (raw)
Alternative zippering as an on-off switch for SNARE-mediated fusion
Claudio G Giraudo et al. Science. 2009.
Abstract
Membrane fusion between vesicles and target membranes involves the zippering of a four-helix bundle generated by constituent helices derived from target- and vesicle-soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs). In neurons, the protein complexin clamps otherwise spontaneous fusion by SNARE proteins, allowing neurotransmitters and other mediators to be secreted when and where they are needed as this clamp is released. The membrane-proximal accessory helix of complexin is necessary for clamping, but its mechanism of action is unknown. Here, we present experiments using a reconstituted fusion system that suggest a simple model in which the complexin accessory helix forms an alternative four-helix bundle with the target-SNARE near the membrane, preventing the vesicle-SNARE from completing its zippering.
Figures
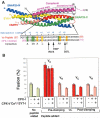
FIGURE 1. CPX-I clamping mechanism
A-top): Cartoon of the 3-D structure of the CPX-SNARE complex (9). Actual structure of CPX-I spans from residues 32-72. CPX-I region 26-32 was modeled as α-helix according to secondary structure predictions. A-bottom): Amino acid sequence alignment of the membrane proximal half of the VAMP2 SNARE motif and the inverted sequence of the accessory a-helical region highlighting identical residues in yellow, conserved residues in light blue and similar residues in green. The hydrophobic layers are also indicated in red blue. Arrows point to the missing hydrophobic layer on CPX-I sequence and the corresponding site-directed mutation performed. B)- VC-peptide but not VN-peptide competes with CPX-I-GPI. v-SNARE cells transfected with YFP-nls and CPX-I-GPI, or YFP-nls, CPX-I-GPI and SYT-I or YFP-nls alone (control) were used for fusion experiments. 30 μM of VC-Peptide or VN-Peptide were added to the reaction before the commencement of the reaction (Pre-clamped) or added only during the fusion recovery with SYT-I/Ca (Post-clamped), and their corresponding effect was measured as a percentage of fusion. Results are mean ± SEM of three independent experiments.
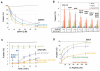
FIGURE 2. Mutations on CPX-I designed to mimic hydrophobic layers on VAMP2 stabilize the clamp
A) Dose dependent inhibition of the cell fusion reaction using different soluble CPX-I mutants (sCPX-I). Increasing concentration of each recombinant sCPX were added at the time the two cell populations were mixed. Cells were allowed to fuse overnight and the fusion efficiency was determined as the percentage of fusion. Results are mean ± SEM of three independent experiments. B) Effect of different cell surface expressed “super-clamp” CPX-I-GPI mutants on cell fusion (blue bars), on the cell fusion recovery after addition of PI-PLC in the absence (green bars) or presence of SYT-I and Calcium (red bars). Experiments are the mean±SEM of three independent experiments. Dashed lines show the maximum cell fusion recovery in the absence (green) or presence of Ca/SYT-I (red) and the total overnight clamping (blue). C) The SYT-I requirement of CPX-I-D27l/E34F-GPI was tested by performing a cell fusion experiment as described in Fig. 2B. In this case the cell fusion recovery was carried out at 200 μM Free Ca2+ and samples were fixed at the indicated time every 5 min. The level of fusion was determined as percentage of transfected v-cells that fused. Results are mean ± SEM of three independent experiments. D) Differential VC-Peptide sensitivity of CPX-I “super-clamp” mutant constructs. Increasing concentrations of VC-peptide were added at the time the two cell populations were mixed. Cells were allowed to fuse overnight and the fusion efficiency was determined as the percentage of fusion. Dashed lines correspond to the basal level of overnight fusion in the absence of CPX-I (green) or in the presence of CPX-I (red). Results are mean ± SEM of three independent experiments
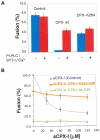
FIGURE 3. Clamping role of the conserved amino acid, K26, in the accessory helix of CPX-I
A)- Effect of CPX-I-K26AGPI-mutant construct on cell fusion (blue bars), and on the efficiency of cell fusion recovery after addition of PI-PLC in the presence of SYT-I and Calcium (red bars). B)- Dose dependent inhibition of the cell fusion reaction using soluble CPX-I (sCPX-I) and flipped-SNARE expressing cells co-transfected with the indicated CPX-I-GPI mutant construct or mock-transfected (control). Experiments are the mean ± SEM of three independent experiments.
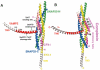
FIGURE 4. Model of the proposed mechanism of clamping of an exocytic SNAREpin by complexin
showing a hypothetical alternative 3-D structure of the clamped state. The 3-D structure of the CPX-cisSNARE complex (9) was modified in accord with super-clamping mutations analyzed in this study. The color code used to label each protein is as follows: CPX (Magenta), VAMP2 VC (red), VAMP2 VN (pink), Syntaxin1 (yellow), the SNAP25 N-terminal helix (green), and SNAP25 C-terminal helix (blue). Membrane anchors are shown as hypothetical helices (grey). The C-terminal end of VAMP2 was displaced from the CPX-cisSNARE structure to accommodate the CPX accessory helix (residues 26-42), which was docked by superimposing CPX Cα positions 34-42 in inverted direction onto VAMP2 Cα positions 69-77. The clamped CPX linker segment (residues 43-52) was built using the Lego-loop feature and regularization in program O (Alwyn Jones). To allow for this clamped CPX docking, the membrane-proximal segment of the VAMP2 helix (residues 60-85) was kinked away from the t-SNARE three-helix bundle after residue 58 by superimposing both this segment and the remaining N-terminal segment onto the AB helix juncture of lamprey hemoglobin (PDB code 2lhb). Precise positioning of the C-terminal portion of VAMP2 (i.e. VC) is arbitrary. The central and accessory helices of CPX-I and the residues involved in the binding with the SNAREs are labeled. The different recognition/binding regions for Botulinum Neurotoxin-B (BoNT/B, residues 62-71) and Tetanus Toxin (TeNT, 38-47) on VAMP2 are indicated as well as their common cleavage site (residue Q76), showing the accessibility of BoNT/B but not of TeNT (12).
Similar articles
- Accessory alpha-helix of complexin I can displace VAMP2 locally in the complexin-SNARE quaternary complex.
Lu B, Song S, Shin YK. Lu B, et al. J Mol Biol. 2010 Feb 26;396(3):602-9. doi: 10.1016/j.jmb.2009.12.020. Epub 2009 Dec 21. J Mol Biol. 2010. PMID: 20026076 Free PMC article. - Complexin controls the force transfer from SNARE complexes to membranes in fusion.
Maximov A, Tang J, Yang X, Pang ZP, Südhof TC. Maximov A, et al. Science. 2009 Jan 23;323(5913):516-21. doi: 10.1126/science.1166505. Science. 2009. PMID: 19164751 Free PMC article. - Interaction of the Complexin Accessory Helix with Synaptobrevin Regulates Spontaneous Fusion.
Vasin A, Volfson D, Littleton JT, Bykhovskaia M. Vasin A, et al. Biophys J. 2016 Nov 1;111(9):1954-1964. doi: 10.1016/j.bpj.2016.09.017. Biophys J. 2016. PMID: 27806277 Free PMC article. - SNARE zippering.
Lou X, Shin YK. Lou X, et al. Biosci Rep. 2016 May 6;36(3):e00327. doi: 10.1042/BSR20160004. Print 2016 Jun. Biosci Rep. 2016. PMID: 27154457 Free PMC article. Review. - Putting the clamps on membrane fusion: how complexin sets the stage for calcium-mediated exocytosis.
Melia TJ Jr. Melia TJ Jr. FEBS Lett. 2007 May 22;581(11):2131-9. doi: 10.1016/j.febslet.2007.02.066. Epub 2007 Mar 5. FEBS Lett. 2007. PMID: 17350005 Review.
Cited by
- Kinetic barriers to SNAREpin assembly in the regulation of membrane docking/priming and fusion.
Li F, Tiwari N, Rothman JE, Pincet F. Li F, et al. Proc Natl Acad Sci U S A. 2016 Sep 20;113(38):10536-41. doi: 10.1073/pnas.1604000113. Epub 2016 Sep 6. Proc Natl Acad Sci U S A. 2016. PMID: 27601655 Free PMC article. - Toward a unified picture of the exocytotic fusion pore.
Karatekin E. Karatekin E. FEBS Lett. 2018 Nov;592(21):3563-3585. doi: 10.1002/1873-3468.13270. Epub 2018 Oct 26. FEBS Lett. 2018. PMID: 30317539 Free PMC article. Review. - Protein trafficking dysfunctions: Role in the pathogenesis of pulmonary arterial hypertension.
Sehgal PB, Lee JE. Sehgal PB, et al. Pulm Circ. 2011 Jan-Mar;1(1):17-32. doi: 10.4103/2045-8932.78097. Pulm Circ. 2011. PMID: 22034594 Free PMC article. - Re-examining how complexin inhibits neurotransmitter release.
Trimbuch T, Xu J, Flaherty D, Tomchick DR, Rizo J, Rosenmund C. Trimbuch T, et al. Elife. 2014 May 8;3:e02391. doi: 10.7554/eLife.02391. Elife. 2014. PMID: 24842998 Free PMC article. - Membrane curvature sensing by the C-terminal domain of complexin.
Snead D, Wragg RT, Dittman JS, Eliezer D. Snead D, et al. Nat Commun. 2014 Sep 17;5:4955. doi: 10.1038/ncomms5955. Nat Commun. 2014. PMID: 25229806 Free PMC article.
References
- Sollner T, et al. Nature. 1993;362:318. - PubMed
- Weber T, et al. Cell. 1998;92:759. - PubMed
- Li F, et al. Nat Struct Mol Biol. 2007;14:890. - PubMed
- Hua SY, Charlton MP. Nat Neurosci. 1999;2:1078. - PubMed
- Wojcik SM, Brose N. Neuron. 2007;55:11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources