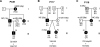Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease - PubMed (original) (raw)
doi: 10.1038/ki.2008.686. Epub 2009 Jan 21.
Vickie J Kubly, Mark B Consugar, Katharina Hopp, Sushmita Roy, Sharon W Horsley, Dominique Chauveau, Lesley Rees, T Martin Barratt, William G van't Hoff, Patrick Niaudet, Vicente E Torres, Peter C Harris
Affiliations
- PMID: 19165178
- PMCID: PMC2813773
- DOI: 10.1038/ki.2008.686
Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease
Sandro Rossetti et al. Kidney Int. 2009 Apr.
Erratum in
- Kidney Int. 2009 Jun;75(12):1359
- Kidney Int. 2010 Feb;77(4):368. Niaudet, W Patrick [corrected to Niaudet, Patrick]
- Correction to "Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease".
[No authors listed] [No authors listed] Kidney Int. 2009 Jun 2;75(12):1359. doi: 10.1038/ki.2009.151. Epub 2015 Oct 20. Kidney Int. 2009. PMID: 30036924 No abstract available.
Abstract
Autosomal dominant polycystic kidney disease (ADPKD) caused by mutations in PKD1 is significantly more severe than PKD2. Typically, ADPKD presents in adulthood but is rarely diagnosed in utero with enlarged, echogenic kidneys. Somatic mutations are thought crucial for cyst development, but gene dosage is also important since animal models with hypomorphic alleles develop cysts, but are viable as homozygotes. We screened for mutations in PKD1 and PKD2 in two consanguineous families and found PKD1 missense variants predicted to be pathogenic. In one family, two siblings homozygous for R3277C developed end stage renal disease at ages 75 and 62 years, while six heterozygotes had few cysts. In the other family, the father and two children with moderate to severe disease were homozygous for N3188S. In both families homozygous disease was associated with small cysts of relatively uniform size while marked cyst heterogeneity is typical of ADPKD. In another family, one patient diagnosed in childhood was found to be a compound heterozygote for the PKD1 variants R3105W and R2765C. All three families had evidence of developmental defects of the collecting system. Three additional ADPKD families with in utero onset had a truncating mutation in trans with either R3277C or R2765C. These cases suggest the presence of incompletely penetrant PKD1 alleles. The alleles alone may result in mild cystic disease; two such alleles cause typical to severe disease; and, in combination with an inactivating allele, are associated with early onset disease. Our study indicates that the dosage of functional PKD1 protein may be critical for cyst initiation.
Figures
Figure 1. Homozygous inheritance of PKD1: R3277C in a consanguineous family
(a) Pedigree of family M34 showed inheritance of PKD through four generations, but only II2 and II3 (black shading) have renal impairment; age at ESRD is shown. Individuals with mild disease are shaded and total cyst number detected by CT or magnetic resonance imaging at the indicated age are shown, including multiple cysts (MC) in I1 at autopsy. Segregation of the R3277C alleles (R or C) is indicated below each patient where information is available, parenthesis indicate inferred genotypes. (b) (II2) Unenhanced coronal MR image (top) and axial MR image (bottom) following administration of gadolinium and (II3) unenhanced CT analysis shows bilaterally enlarged kidneys with numerous small and uniform cysts. Following the administration of gadolinium, there is layering of the contrast (white arrows) in dilated calyces (II2). Single cysts are indicated with arrowheads on CT (III1 and III2) or magnetic resonance imaging (III4) of mildly affected individuals. (c) Wild-type (wt), heterozygous (het), and homozygous (hom) sequence of R3277C showing the normal and variant DNA and amino-acid sequence. (d) Multisequence alignment of PC1 orthologues as indicated shows R3277 is invariant. PC1-like human sequences, as indicated, illustrate a high level of conservation of R3277.
Figure 2. Homozygous inheritance of PKD1: N3188S in a consanguineous Pakistani family
(a) Pedigree showing renal phenotype; multiple cysts (MC), in utero (IU) PKD, or negative ultrasound (–ve U/S) and the genotype of N3188S (N or S). (b) Sequence of N3188S showing the wild-type (wt), heterozygous (het), and homozygous (hom) DNA and amino-acid sequence. The position of IVS27 is also indicated. (c) Sequence alignment of PC1 orthologues and human PC1-like proteins as indicated. N3188 is completely conserved in the orthologues and well conserved in homologues, but is serine in PC1L1.
Figure 3. Family M390-inherited PKD1 variants: R2765C and R3105W
(a) Pedigree of M390 showing the proband II5, who has multiple cysts (MC) in the kidney, and mildly affected individuals (II2 and II3) with single cysts; negative imaging data and age are illustrated. The R2765C (R or C) and R3105W (R or W) genotypes are shown below. Only II5 is a compound heterozygote. (b) CT images of II5 kidney (top and middle) showing multiple renal cysts and (bottom) a single liver cyst (arrowheads). (c) CT of II2 showing a single large cyst. (d) Sequence data illustrating the two variants showing the nucleotide and amino-acid changes. (e) Sequence alignment of PC1 orthologues and human PC1-like homologous proteins. R3105 is highly conserved as a basic residue in all homologues. (f) Multisequence alignment of PC1 orthologues shows that R2765 is a basic residue in all species.
Figure 4. Pedigrees of three families with in utero ADPKD presentations that have inherited a truncating and a hypomorphic ADPKD allele
Each family has an in utero (IU) case, and renal phenotypes of other family members are shown: multiple cysts (MC); negative ultrasound (–ve U/S), or ESRD. Genotypes of the PKD1 variants: a, Q2158X and R3227C; b, Y3819X and R2765C; and c, 7915dup20 and R2765C, are shown below each pedigree. The etiology of cysts in P118 I1 is unclear but could potentially represent a low level of mosaicism not detected by sequence analysis.
Comment in
- The diversity of PKD1 alleles: implications for disease pathogenesis and genetic counseling.
Sandford RN. Sandford RN. Kidney Int. 2009 Apr;75(8):765-7. doi: 10.1038/ki.2009.17. Kidney Int. 2009. PMID: 19337214 Review.
Similar articles
- Autosomal dominant polycystic kidney disease in a family with mosaicism and hypomorphic allele.
Reiterová J, Štekrová J, Merta M, Kotlas J, Elišáková V, Lněnička P, Korabečná M, Kohoutová M, Tesař V. Reiterová J, et al. BMC Nephrol. 2013 Mar 15;14:59. doi: 10.1186/1471-2369-14-59. BMC Nephrol. 2013. PMID: 23496908 Free PMC article. - Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD.
Vujic M, Heyer CM, Ars E, Hopp K, Markoff A, Orndal C, Rudenhed B, Nasr SH, Torres VE, Torra R, Bogdanova N, Harris PC. Vujic M, et al. J Am Soc Nephrol. 2010 Jul;21(7):1097-102. doi: 10.1681/ASN.2009101070. Epub 2010 Jun 17. J Am Soc Nephrol. 2010. PMID: 20558538 Free PMC article. - Modifiers of Autosomal Dominant Polycystic Kidney Disease Severity: The Role of PKD1 Hypomorphic Alleles.
Ambrosini E, Montanari F, Cristalli CP, Capelli I, La Scola C, Pasini A, Graziano C. Ambrosini E, et al. Genes (Basel). 2023 Jun 7;14(6):1230. doi: 10.3390/genes14061230. Genes (Basel). 2023. PMID: 37372410 Free PMC article. Review. - Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity.
Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, Rossetti S, Torres VE, Harris PC. Hopp K, et al. J Clin Invest. 2012 Nov;122(11):4257-73. doi: 10.1172/JCI64313. Epub 2012 Oct 15. J Clin Invest. 2012. PMID: 23064367 Free PMC article. - The diversity of PKD1 alleles: implications for disease pathogenesis and genetic counseling.
Sandford RN. Sandford RN. Kidney Int. 2009 Apr;75(8):765-7. doi: 10.1038/ki.2009.17. Kidney Int. 2009. PMID: 19337214 Review.
Cited by
- New Mutation Associated with Polycystic Kidney Disease Type I: A Case Report.
Rai V, Singh M, Holthoff JH. Rai V, et al. Genes (Basel). 2024 Sep 27;15(10):1262. doi: 10.3390/genes15101262. Genes (Basel). 2024. PMID: 39457385 Free PMC article. - A guide to gene-disease relationships in nephrology.
Stark Z, Byrne AB, Sampson MG, Lennon R, Mallett AJ. Stark Z, et al. Nat Rev Nephrol. 2024 Oct 23. doi: 10.1038/s41581-024-00900-7. Online ahead of print. Nat Rev Nephrol. 2024. PMID: 39443743 Review. - Activation of polycystin-1 signaling by binding of stalk-derived peptide agonists.
Pawnikar S, Magenheimer BS, Joshi K, Nevarez-Munoz E, Haldane A, Maser RL, Miao Y. Pawnikar S, et al. Elife. 2024 Oct 7;13:RP95992. doi: 10.7554/eLife.95992. Elife. 2024. PMID: 39373641 Free PMC article. - The Impact of Autosomal Dominant Polycystic Kidney Disease in Children: A Nephrological, Nutritional, and Psychological Point of View.
Guarnaroli M, Padoan F, Fava C, Benetti MG, Brugnara M, Pietrobelli A, Piacentini G, Pecoraro L. Guarnaroli M, et al. Biomedicines. 2024 Aug 12;12(8):1823. doi: 10.3390/biomedicines12081823. Biomedicines. 2024. PMID: 39200287 Free PMC article. Review. - Cross-Species Insights into Autosomal Dominant Polycystic Kidney Disease: Provide an Alternative View on Research Advancement.
Luo J, Zhang Y, Jayaprakash S, Zhuang L, He J. Luo J, et al. Int J Mol Sci. 2024 May 22;25(11):5646. doi: 10.3390/ijms25115646. Int J Mol Sci. 2024. PMID: 38891834 Free PMC article. Review.
References
- European Polycystic Kidney Disease Consortium The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. Cell. 1994;77:881–894. - PubMed
- Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. - PubMed
- Rossetti S, Consugar MB, Chapman AB, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18:2143–2160. - PubMed
- Rossetti S, Harris PC. Genotype–phenotype correlations in autosomal dominant and autosomal recessive polycystic kidney disease. J Am Soc Nephrol. 2007;18:1374–1380. - PubMed
- Hateboer N, van Dijk MA, Bogdanova N, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet. 1999;353:103–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous