A systematic approach to mapping recessive disease genes in individuals from outbred populations - PubMed (original) (raw)
. 2009 Jan;5(1):e1000353.
doi: 10.1371/journal.pgen.1000353. Epub 2009 Jan 23.
Saskia F Heeringa, Franz Rüschendorf, Massimo Attanasio, Gudrun Nürnberg, Christian Becker, Dominik Seelow, Norbert Huebner, Gil Chernin, Christopher N Vlangos, Weibin Zhou, John F O'Toole, Bethan E Hoskins, Matthias T F Wolf, Bernward G Hinkes, Hassan Chaib, Shazia Ashraf, Dominik S Schoeb, Bugsu Ovunc, Susan J Allen, Virginia Vega-Warner, Eric Wise, Heather M Harville, Robert H Lyons, Joseph Washburn, James Macdonald, Peter Nürnberg, Edgar A Otto
Affiliations
- PMID: 19165332
- PMCID: PMC2621355
- DOI: 10.1371/journal.pgen.1000353
A systematic approach to mapping recessive disease genes in individuals from outbred populations
Friedhelm Hildebrandt et al. PLoS Genet. 2009 Jan.
Abstract
The identification of recessive disease-causing genes by homozygosity mapping is often restricted by lack of suitable consanguineous families. To overcome these limitations, we apply homozygosity mapping to single affected individuals from outbred populations. In 72 individuals of 54 kindred ascertained worldwide with known homozygous mutations in 13 different recessive disease genes, we performed total genome homozygosity mapping using 250,000 SNP arrays. Likelihood ratio Z-scores (ZLR) were plotted across the genome to detect ZLR peaks that reflect segments of homozygosity by descent, which may harbor the mutated gene. In 93% of cases, the causative gene was positioned within a consistent ZLR peak of homozygosity. The number of peaks reflected the degree of inbreeding. We demonstrate that disease-causing homozygous mutations can be detected in single cases from outbred populations within a single ZLR peak of homozygosity as short as 2 Mb, containing an average of only 16 candidate genes. As many specialty clinics have access to cohorts of individuals from outbred populations, and as our approach will result in smaller genetic candidate regions, the new strategy of homozygosity mapping in single outbred individuals will strongly accelerate the discovery of novel recessive disease genes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
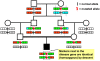
Figure 1. Homozygosity mapping of recessive disease genes.
An individual with an autosomal recessive disease (filled symbol) whose parents are consanguineous will very likely be homozygous (identical) by descent for the disease allele (“mutated allele”), because a rare mutation may segregate from a common ancestor to the child through both the father's and mother's line, rendering the child homozygous for the mutation. Chromosomal segments surrounding the disease gene locus are shown here with 3 marker positions to either side. Different marker alleles are represented by different colors. Although for each parent-offspring succession there is an opportunity for a crossing over (dotted line) to occur in the parents' gametes, there is a high likelihood that in the affected child consecutive markers surrounding the mutation will not have recombined and will be identical (homozygous) by descent. This segment of homozygous markers (dotted box) can be detected as a “cZLR” peak in multipoint evaluation (see Figure S1 and Figure S2), leading to successful mapping of the disease gene. Note also that more remote consanguinity will give more opportunities for crossing over to occur and will thereby lead in the affected individual to fewer and shorter homozygous intervals that contain the disease gene (see Figures S2 and S3).
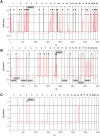
Figure 2. Homozygous mutations in recessive disease genes are positioned in cZLR peaks.
Homozygosity profiles (see Figure S1 and Methods) are shown for individuals who have a known homozygous mutation in a recessive disease gene causing nephronophthisis (NPHP). Individuals are (A) from a consanguineous marriage, (B) without known consanguinity but originating from a geographic population with frequent consanguinity, and (C) originating from an outbred population. Non-parametric LOD scores were calculated with ALLEGRO assuming first-degree cousin consanguinity of the parents, regardless of actual consanguinity status. ZLR scores (minor allele frequency >0.3) were plotted over genetic distance across the genome, where chromosomal positions are concatenated from p- to q-arm (left to right). cZLR peaks (arrow heads) exceeding the empirical cut-off value of 2.0 (dotted line) as described in Figure S1 reflect possible segments of homozygosity by descent, one of which harbors the homozygous disease gene mutation in each patient (arrows with flags indicate the position of the mutated gene). (A) Individual A14-2 exhibits 28 cZLR peaks. This individual has a known homozygous NPHP3 mutation (flagged position) and is from a consanguineous Venezuelan kindred with 17 documented consanguinity loops . (B) Individual A8-1 exhibits 12 cZLR peaks. This family is not aware of consanguinity but originates from a geographic region where consanguinity is frequent (Turkey). Note that the homozygosity profile permits exclusion of homozygous mutations from 8 different NPHP loci (NPHP1 and NPHP3-9), which are marked by vertical dashed lines flagged with gene name at the bottom, and directs mutation analysis to NPHP2, in which the individual carried the disease-causing homozygous mutation. (C) Individual F399-1 exhibits only 1 cZLR peak of 8.25 Mb width, which harbors the disease causing homozygous mutation of NPHP5. This individual originates from a geographic region where consanguinity is rare (Germany).
Figure 3. Detection limit for recessive disease loci in 72 affected individuals of 54 families with known homozygous disease-causing mutations.
Total genome search by homozygosity mapping was performed by typing 250 K SNP arrays in 72 individuals of 54 families with known homozygous mutations in 13 different autosomal recessive kidney disease genes. Under the hypothesis of identity by descent, total genome homozygosity profiles were calculated as described in Methods. The numbers of cZLR peaks (Figure S1) were plotted per family. Families were ordered from the highest to the lowest number of cZLR peaks from left to right. On the X-axis orange highlighting is used for 15 families with known consanguinity, yellow highlighting for 12 families with no known consanguinity but originating from a population with frequent consanguinity, and no highlighting (white) for 27 families from outbred populations. Family number is preceded by one or two asterisks if data were generated by 50 K or 10 K SNP arrays, respectively. Filled circles denote affected individuals with homozygous mutations (siblings numbered as 1, 2, or 3 to the left or right), open circles denote father (F) or mother (M). Dashes denote number of peaks for two affected siblings calculated together (individual numbers shown above dashes), filled triangles for three siblings calculated together (individual numbers shown below triangle). Note that families with known consanguinity (orange) cluster in the left third of the plot with cZLR peaks ranging from 60 to 8, families without known consanguinity but from a populations, in which consanguinity is frequent (yellow) cluster in the middle with number of cZLR peaks ranging from 8 to 4, whereas families from outbred populations (no highlighting) cluster in the right third of the plot with cZLR peaks ranging from 4 to 0. Inset: In families from outbred populations that yielded 2 or fewer cZLR peaks, cumulative physical width in Mb for all cZLR peaks together is plotted (red, with axis on the right; exponential fit; if no peak was detected extent of homozygous markers at the gene locus is given). The cut off at which no cZLR peak was detectable was for a cZLR peak width of 2.1 Mb (see also Table 1). Note that parents of family F30_10G are known to be related 10 generations back, and that F190_HET represents an outbred control individual with a known compound heterozygous mutation in MKS3.
Similar articles
- Identification of mutations causing inherited retinal degenerations in the israeli and palestinian populations using homozygosity mapping.
Beryozkin A, Zelinger L, Bandah-Rozenfeld D, Shevach E, Harel A, Storm T, Sagi M, Eli D, Merin S, Banin E, Sharon D. Beryozkin A, et al. Invest Ophthalmol Vis Sci. 2014 Feb 24;55(2):1149-60. doi: 10.1167/iovs.13-13625. Invest Ophthalmol Vis Sci. 2014. PMID: 24474277 - Homozygosity mapping in outbred families with mental retardation.
Schuurs-Hoeijmakers JH, Hehir-Kwa JY, Pfundt R, van Bon BW, de Leeuw N, Kleefstra T, Willemsen MA, van Kessel AG, Brunner HG, Veltman JA, van Bokhoven H, de Brouwer AP, de Vries BB. Schuurs-Hoeijmakers JH, et al. Eur J Hum Genet. 2011 May;19(5):597-601. doi: 10.1038/ejhg.2010.167. Epub 2011 Jan 19. Eur J Hum Genet. 2011. PMID: 21248743 Free PMC article. - Pitfalls in homozygosity mapping.
Miano MG, Jacobson SG, Carothers A, Hanson I, Teague P, Lovell J, Cideciyan AV, Haider N, Stone EM, Sheffield VC, Wright AF. Miano MG, et al. Am J Hum Genet. 2000 Nov;67(5):1348-51. doi: 10.1016/S0002-9297(07)62966-8. Epub 2000 Sep 27. Am J Hum Genet. 2000. PMID: 11007652 Free PMC article. - Discovery of rare homozygous mutations from studies of consanguineous pedigrees.
Alkuraya FS. Alkuraya FS. Curr Protoc Hum Genet. 2012 Oct;Chapter 6:Unit6.12. doi: 10.1002/0471142905.hg0612s75. Curr Protoc Hum Genet. 2012. PMID: 23074070 Review. - Importance of Genetic Studies in Consanguineous Populations for the Characterization of Novel Human Gene Functions.
Erzurumluoglu AM, Shihab HA, Rodriguez S, Gaunt TR, Day IN. Erzurumluoglu AM, et al. Ann Hum Genet. 2016 May;80(3):187-96. doi: 10.1111/ahg.12150. Epub 2016 Mar 22. Ann Hum Genet. 2016. PMID: 27000383 Free PMC article. Review.
Cited by
- Mutation of the Mg2+ transporter SLC41A1 results in a nephronophthisis-like phenotype.
Hurd TW, Otto EA, Mishima E, Gee HY, Inoue H, Inazu M, Yamada H, Halbritter J, Seki G, Konishi M, Zhou W, Yamane T, Murakami S, Caridi G, Ghiggeri G, Abe T, Hildebrandt F. Hurd TW, et al. J Am Soc Nephrol. 2013 May;24(6):967-77. doi: 10.1681/ASN.2012101034. Epub 2013 May 9. J Am Soc Nephrol. 2013. PMID: 23661805 Free PMC article. - IFT81, encoding an IFT-B core protein, as a very rare cause of a ciliopathy phenotype.
Perrault I, Halbritter J, Porath JD, Gérard X, Braun DA, Gee HY, Fathy HM, Saunier S, Cormier-Daire V, Thomas S, Attié-Bitach T, Boddaert N, Taschner M, Schueler M, Lorentzen E, Lifton RP, Lawson JA, Garfa-Traore M, Otto EA, Bastin P, Caillaud C, Kaplan J, Rozet JM, Hildebrandt F. Perrault I, et al. J Med Genet. 2015 Oct;52(10):657-65. doi: 10.1136/jmedgenet-2014-102838. Epub 2015 Aug 14. J Med Genet. 2015. PMID: 26275418 Free PMC article. - Mutations of ADAMTS9 Cause Nephronophthisis-Related Ciliopathy.
Choi YJ, Halbritter J, Braun DA, Schueler M, Schapiro D, Rim JH, Nandadasa S, Choi WI, Widmeier E, Shril S, Körber F, Sethi SK, Lifton RP, Beck BB, Apte SS, Gee HY, Hildebrandt F. Choi YJ, et al. Am J Hum Genet. 2019 Jan 3;104(1):45-54. doi: 10.1016/j.ajhg.2018.11.003. Am J Hum Genet. 2019. PMID: 30609407 Free PMC article. - Further host-genomic characterization of total antibody response to PRRSV vaccination and its relationship with reproductive performance in commercial sows: genome-wide haplotype and zygosity analyses.
Sanglard LP, Huang Y, Gray KA, Linhares DCL, Dekkers JCM, Niederwerder MC, Fernando RL, Serão NVL. Sanglard LP, et al. Genet Sel Evol. 2021 Dec 7;53(1):91. doi: 10.1186/s12711-021-00676-5. Genet Sel Evol. 2021. PMID: 34875996 Free PMC article. - The application of genome-wide SNP genotyping methods in studies on livestock genomes.
Gurgul A, Semik E, Pawlina K, Szmatoła T, Jasielczuk I, Bugno-Poniewierska M. Gurgul A, et al. J Appl Genet. 2014 May;55(2):197-208. doi: 10.1007/s13353-014-0202-4. Epub 2014 Feb 25. J Appl Genet. 2014. PMID: 24566962 Review.
References
- Boute N, Gribouval O, Roselli S, Benessy F, Lee H, et al. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet. 2000;24:349–354. - PubMed
- Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, et al. Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol. 2004;15:722–732. - PubMed
- Hinkes BG, Mucha B, Vlangos CN, Gbadegesin R, Liu J, et al. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics. 2007;119:e907–919. - PubMed
- Lander ES, Botstein D. Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science. 1987;236:1567–1570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK068306/DK/NIDDK NIH HHS/United States
- DK076683/DK/NIDDK NIH HHS/United States
- HD045345/HD/NICHD NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 DK076683/DK/NIDDK NIH HHS/United States
- DK069274/DK/NIDDK NIH HHS/United States
- R01 DK068306/DK/NIDDK NIH HHS/United States
- R01 DK069274/DK/NIDDK NIH HHS/United States
- DK064614/DK/NIDDK NIH HHS/United States
- R01 DK064614/DK/NIDDK NIH HHS/United States
- R01 HD045345/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous