Mitochondrial dysfunction contributes to alveolar developmental arrest in hyperoxia-exposed mice - PubMed (original) (raw)
Mitochondrial dysfunction contributes to alveolar developmental arrest in hyperoxia-exposed mice
Veniamin Ratner et al. Am J Respir Cell Mol Biol. 2009 May.
Abstract
This study investigated whether mitochondrial dysfunction contributes to alveolar developmental arrest in a mouse model of bronchopulmonary dysplasia (BPD). To induce BPD, 3-day-old mice were exposed to 75% O2. Mice were studied at two time points of hyperoxia (72 h or 2 wk) and after 3 weeks of recovery in room air (RA). A separate cohort of mice was exposed to pyridaben, a complex-I (C-I) inhibitor, for 72 hours or 2 weeks. Alveolarization was quantified by radial alveolar count and mean linear intercept methods. Pulmonary mitochondrial function was defined by respiration rates, ATP-production rate, and C-I activity. At 72 hours, hyperoxic mice demonstrated significant inhibition of C-I activity, reduced respiration and ATP production rates, and significantly decreased radial alveolar count compared with controls. Exposure to pyridaben for 72 hours, as expected, caused significant inhibition of C-I and ADP-phosphorylating respiration. Similar to hyperoxic littermates, these pyridaben-exposed mice exhibited significantly delayed alveolarization compared with controls. At 2 weeks of exposure to hyperoxia or pyridaben, mitochondrial respiration was inhibited and associated with alveolar developmental arrest. However, after 3 weeks of recovery from hyperoxia or 2 weeks after 72 hours of exposure to pyridaben alveolarization significantly improved. In addition, there was marked normalization of C-I and mitochondrial respiration. The degree of hyperoxia-induced pulmonary simplification and recovery strongly (r(2) = 0.76) correlated with C-I activity in lung mitochondria. Thus, the arrest of alveolar development induced by either hyperoxia or direct inhibition of mitochondrial oxidative phosphorylation indicates that bioenergetic failure to maintain normal alveolar development is one of the fundamental mechanisms responsible for BPD.
Figures
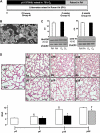
Figure 1.
(A) Experimental timeline and design. (B) Electron microscopy of pulmonary mitochondria isolated from naïve and hyperoxic Postnatal Day (P)18 mice. Scale bar = 500 nm. (C) Immunoblots of representative samples from naïve (room air [RA]-exposed) and O2-exposed mice and optical density graphs of MnSOD and COX IV expression in mitochondrial isolates from naïve P18 mice (open bars, n = 4) and P18 mice subjected to hyperoxia for 2 weeks (solid bars, n = 4). (D and E) Representative images of hematoxylin and eosin–stained lung sections with corresponding radial alveolar count (RAC) analysis; in naïve mice (open bars, P3 n = 4, P6 n = 7, P18 n = 10, P39 n = 4), mice subjected to hyperoxia (solid bars, P6 n = 8, P18 n = 8), and recovery (hatched bar, P39 n = 4). Scale bar = 500 μm. Data are mean ± SEM. *P ≤ 0.002 compared with naïve littermates, **P = 0.0001 compared with naïve P6 mice, #P < 0.0001 compared with P6 and P18 hyperoxia-exposed mice, §P = 0.0003 compared with P3 naïve mice.
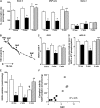
Figure 2.
(A) Pulmonary mitochondrial respiration rates during phosphorylation of ADP (State 3), after acceleration by DNP (DNP-rate), and during resting (State 4) respiration in naïve (open bars) and hyperoxia-exposed mice (solid bars) measured: at 72 hours (naïve n = 4, hyperoxic mice n = 5), at 2 weeks (naïve n = 6, hyperoxic mice n = 7), and after 3 weeks of recovery (hatched bars, naïve = 4, hyperoxic mice n = 4). (B) Representative recording of mitochondrial respiration in a mouse subjected to hyperoxia for 2 weeks (dashed line) and naïve mouse (solid line). Time-points at which mitochondria, ADP, and DNP were added are indicated. (C and D) RCR and ADP:O ratio in mice subjected to hyperoxia for 72 hours, 2 weeks (solid bars), and after 3 weeks of recovery (hatched bar) compared with naïve littermates (open bars). (E) Complex I activity in pulmonary mitochondria isolated at 72 hours of hyperoxia (solid bar, n = 5) compared with naïve mice (open bar, n = 4), at 2 weeks of hyperoxia (solid bar, n = 5) compared with naïve mice (open bar, n = 5), and after 3 weeks of recovery in RA (hatched bar, n = 4) compared with naïve littermates (open bar, n = 4). (F) Correlation between RAC and C-I activity in mice subjected to hyperoxia for 2 weeks (solid circles) and in mice recovered for 3 weeks after 2 weeks of hyperoxia (hatched circles). All data are mean ± SEM. *P < 0.01 compared with naïve littermates, **P < 0.01 compared with mice subjected to hyperoxia for 2 weeks, ¶P = 0.04 compared with P6 naïve mice, #P = 0.004 compared with mice subjected to hyperoxia for 72 hours, *P = 0.0002 compared with mice exposed to hyperoxia for 72 hours.
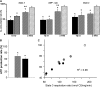
Figure 3.
(A) Respiration rates in pulmonary mitochondria isolated from mice subjected to hyperoxia (solid bar, n = 8), pyridaben (hatched bar, n = 6) for 72 hours or 2 weeks (n = 5 in each group) compared with mice recovered from 72 hours of pyridaben exposure for 2 weeks (bricked bar, n = 6). Data expressed as percentage of mean values (100%) in vehicle-treated littermates (n = 5 for each age group). *P < 0.05 compared with vehicle controls, **P < 0.01 compared with 72 hours of pyridaben exposure. (B) ATP-production rate in mitochondria after 72 hours of exposure to pyridaben (hatched bar, n = 4) or hyperoxia (solid bar, n = 5). Data expressed as percentage of mean value (100%) in vehicle-treated littermates. (C) Correlation between phosphrylating respiration and ATP production rates: open circle, the mean value of State 3 respiration rate in vehicle-treated mice (n = 4); hatched circles, pyridaben; solid circles, hyperoxia-exposed mice. All data are mean ± SEM. *P ≤ 0.03 compared with vehicle-treated controls.
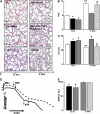
Figure 4.
(A) Representative images of lung sections in mice exposed to hyperoxia (O2), pyridaben for 72 hours or 2 weeks, and mice allowed to recover after 72 hours of pyridaben exposure compared with age-matched vehicle-treated controls. Scale bar = 500 μm. (B and C) RAC and Lm in mice subjected to hyperoxia (solid bar, n = 8), pyridaben (hatched bar, n = 6) for 72 hours or 2 weeks (n = 5), and in mice after recovery from 72 hours of pyridaben exposure (bricked bar, n = 6) compared with vehicle-treated controls (open bar, n = 4 and n = 6). (D) Recording of mitochondrial respiration in mouse subjected to pyridaben for 2 weeks (dashed line) and vehicle-treated littermate (solid line). Time points at which mitochondria and ADP were added, and end-points of ATP production, are indicated. (E) ADP:O ratio (% of mean value in vehicle-treated controls, n = 6) in mitochondria from mice exposed to hyperoxia for 72 hours (solid bar, n = 5), pyridaben (hatched bar, at 72 h n = 6 and at 2 wk n = 5), and mice recovering from 72 hours of pyridaben exposure (bricked bar, n = 6). All data are mean ± SEM. *P ≤ 0.002 compared with age-matched vehicle-treated controls, **P ≤ 0.03 compared with P6 vehicle-treated controls, §P < 0.04 compared with mice exposed to pyridabed for 2 weeks.
Similar articles
- Mechanical ventilation causes pulmonary mitochondrial dysfunction and delayed alveolarization in neonatal mice.
Ratner V, Sosunov SA, Niatsetskaya ZV, Utkina-Sosunova IV, Ten VS. Ratner V, et al. Am J Respir Cell Mol Biol. 2013 Dec;49(6):943-50. doi: 10.1165/rcmb.2012-0172OC. Am J Respir Cell Mol Biol. 2013. PMID: 23980609 Free PMC article. - Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure.
Velten M, Heyob KM, Rogers LK, Welty SE. Velten M, et al. J Appl Physiol (1985). 2010 May;108(5):1347-56. doi: 10.1152/japplphysiol.01392.2009. Epub 2010 Mar 11. J Appl Physiol (1985). 2010. PMID: 20223995 Free PMC article. - Mitochondrial DNA variation modulates alveolar development in newborn mice exposed to hyperoxia.
Kandasamy J, Rezonzew G, Jilling T, Ballinger S, Ambalavanan N. Kandasamy J, et al. Am J Physiol Lung Cell Mol Physiol. 2019 Dec 1;317(6):L740-L747. doi: 10.1152/ajplung.00220.2019. Epub 2019 Aug 21. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 31432715 Free PMC article. - Cumulative effects of neonatal hyperoxia on murine alveolar structure and function.
Cox AM, Gao Y, Perl AT, Tepper RS, Ahlfeld SK. Cox AM, et al. Pediatr Pulmonol. 2017 May;52(5):616-624. doi: 10.1002/ppul.23654. Epub 2017 Feb 10. Pediatr Pulmonol. 2017. PMID: 28186703 Free PMC article. - Hypoxic stress exacerbates hyperoxia-induced lung injury in a neonatal mouse model of bronchopulmonary dysplasia.
Ratner V, Slinko S, Utkina-Sosunova I, Starkov A, Polin RA, Ten VS. Ratner V, et al. Neonatology. 2009;95(4):299-305. doi: 10.1159/000178798. Epub 2008 Dec 4. Neonatology. 2009. PMID: 19052476 Free PMC article.
Cited by
- Coenzyme Q(1) as a probe for mitochondrial complex I activity in the intact perfused hyperoxia-exposed wild-type and Nqo1-null mouse lung.
Bongard RD, Myers CR, Lindemer BJ, Baumgardt S, Gonzalez FJ, Merker MP. Bongard RD, et al. Am J Physiol Lung Cell Mol Physiol. 2012 May 1;302(9):L949-58. doi: 10.1152/ajplung.00251.2011. Epub 2012 Jan 20. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22268123 Free PMC article. - Role of glutathione in lung retention of 99mTc-hexamethylpropyleneamine oxime in two unique rat models of hyperoxic lung injury.
Audi SH, Roerig DL, Haworth ST, Clough AV. Audi SH, et al. J Appl Physiol (1985). 2012 Aug 15;113(4):658-65. doi: 10.1152/japplphysiol.00441.2012. Epub 2012 May 24. J Appl Physiol (1985). 2012. PMID: 22628374 Free PMC article. - Developing chicken cardiac muscle mitochondria are resistant to variations in incubation oxygen levels.
Starr VJ, Dzialowski EM. Starr VJ, et al. Curr Res Physiol. 2022 Mar 17;5:151-157. doi: 10.1016/j.crphys.2022.03.001. eCollection 2022. Curr Res Physiol. 2022. PMID: 35345510 Free PMC article. - Fatty Acid Oxidation Protects against Hyperoxia-induced Endothelial Cell Apoptosis and Lung Injury in Neonatal Mice.
Yao H, Gong J, Peterson AL, Lu X, Zhang P, Dennery PA. Yao H, et al. Am J Respir Cell Mol Biol. 2019 Jun;60(6):667-677. doi: 10.1165/rcmb.2018-0335OC. Am J Respir Cell Mol Biol. 2019. PMID: 30571144 Free PMC article. - Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period.
Perez M, Robbins ME, Revhaug C, Saugstad OD. Perez M, et al. Free Radic Biol Med. 2019 Oct;142:61-72. doi: 10.1016/j.freeradbiomed.2019.03.035. Epub 2019 Apr 5. Free Radic Biol Med. 2019. PMID: 30954546 Free PMC article. Review.
References
- Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007;357:1946–1955. - PubMed
- Van Marter LJ. Progress in discovery and evaluation of treatments to prevent bronchopulmonary dysplasia. Biol Neonate 2006;89:303–312. - PubMed
- Brunton JA, Saigal S, Atkinson SA. Growth and body composition in infants with bronchopulmonary dysplasia up to 3 months corrected age: a randomized trial of a high-energy nutrient-enriched formula fed after hospital discharge. J Pediatr 1998;133:340–345. - PubMed
- Greer FR, McCormick A. Bone growth with low bone mineral content in very low birth weight premature infants. Pediatr Res 1986;20:925–928. - PubMed
- Furman L, Hack M, Watts C, Borawski-Clark E, Baley J, Amini S, Hook B. Twenty-month outcome in ventilator-dependent, very low birth weight infants born during the early years of dexamethasone therapy. J Pediatr 1995;126:434–440. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources