Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children's oncology group study - PubMed (original) (raw)
Randomized Controlled Trial
. 2009 Mar 1;27(7):1007-13.
doi: 10.1200/JCO.2007.13.8925. Epub 2009 Jan 26.
Affiliations
- PMID: 19171716
- PMCID: PMC2738615
- DOI: 10.1200/JCO.2007.13.8925
Randomized Controlled Trial
Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children's oncology group study
Katherine K Matthay et al. J Clin Oncol. 2009.
Erratum in
- J Clin Oncol. 2014 Jun 10;32(17):1862-3
Abstract
PURPOSE We assessed the long-term outcome of patients enrolled on CCG-3891, a high-risk neuroblastoma study in which patients were randomly assigned to undergo autologous purged bone marrow transplantation (ABMT) or to receive chemotherapy, and subsequent treatment with 13-cis-retinoic acid (cis-RA). PATIENTS AND METHODS Patients received the same induction chemotherapy, with random assignment (N = 379) to consolidation with myeloablative chemotherapy, total-body irradiation, and ABMT versus three cycles of intensive chemotherapy. Patients who completed consolidation without disease progression were randomly assigned to receive no further therapy or cis-RA for 6 months. Results The event-free survival (EFS) for patients randomly assigned to ABMT was significantly higher than those randomly assigned to chemotherapy; the 5-year EFS (mean +/- SE) was 30% +/- 4% versus 19% +/- 3%, respectively (P = .04). The 5-year EFS (42% +/- 5% v 31% +/- 5%) from the time of second random assignment was higher for cis-RA than for no further therapy, though it was not significant. Overall survival (OS) was significantly higher for each random assignment by a test of the log(-log(.)) transformation of the survival estimates at 5 years (P < .01). The 5-year OS from the second random assignment of patients who underwent both random assignments and who were assigned to ABMT/cis-RA was 59% +/- 8%; for ABMT/no cis-RA, it was 41% +/- 8% [corrected]; for continuing chemotherapy/cis-RA, it was 38% +/- 7%; and for chemotherapy/no cis-RA, it was 36% +/- 7%.
Conclusion: Myeloablative therapy and autologous hematopoietic cell rescue result in significantly better 5-year EFS than nonmyeloablative chemo therapy; neither myeloablative therapy with [corrected] autologous hematopoietic cell rescue nor cis-RA given after consolidation therapy significantly improved OS.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
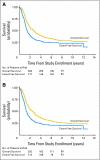
Fig 1.
(A) Event-free survival (EFS) and overall survival (OS) for all patients (N = 539). (B) EFS and OS for patients with stage 4 disease (n = 466).
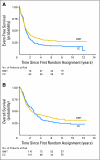
Fig 2.
(A) Event-free survival for patients randomly assigned to continuing chemotherapy (CC; n = 190) versus autologous bone marrow transplantation (BMT; n = 189). P = .0434. (B) Overall survival for patients randomly assigned to CC (n = 190) versus BMT (n = 189). P = .3917 by log-rank test; P < .0001 by test of the log(−log(.)) transformation of the survival estimates at 5 years.
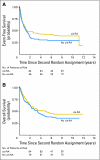
Fig 3.
(A) Event-free survival for patients randomly assigned to 13-_cis_-retinoic acid (_cis_-RA) (n = 130) versus no _cis_-RA (n = 128). P = .1219. (B) Overall survival for patients randomly asigned to _cis_-RA (n = 130) versus no _cis_-RA (n = 128). P = .1946 by log-rank test; P = .0006 by test of the log(−log(.)) transformation of the survival estimates at 5 years.
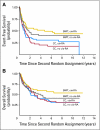
Fig 4.
(A) Event-free survival for patients who participated in both the first and second random assignments (autologous bone marrow transplantation + 13-_cis_-retinoic acid [_cis_-RA] [n = 50] versus continuing chemotherapy (CC) + no _cis_-RA [n = 53]). P = .0038. (B) Overall survival for patients who participated in both the first and second random assignments (autologous bone marrow transportation + _cis_-RA versus CC + no _cis_-RA) P = .0540.
Comment in
- Reply to N.-K.V. Cheung et al.
Matthay KK, London WB, Maris J, Adamson PC, Park JR. Matthay KK, et al. J Clin Oncol. 2014 Dec 20;32(36):4174-5. doi: 10.1200/JCO.2014.58.7006. Epub 2014 Nov 17. J Clin Oncol. 2014. PMID: 25403223 Free PMC article. No abstract available. - When overall survival fails to confirm event-free survival, should the latter be used to set the standard of care?
Cheung NK, Modak S, Ostrovnaya I, Roberts SS, Basu EM, Kramer K, Kushner BH. Cheung NK, et al. J Clin Oncol. 2014 Dec 20;32(36):4173-4. doi: 10.1200/JCO.2014.58.1678. Epub 2014 Nov 17. J Clin Oncol. 2014. PMID: 25403224 No abstract available.
Similar articles
- Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group.
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP. Matthay KK, et al. N Engl J Med. 1999 Oct 14;341(16):1165-73. doi: 10.1056/NEJM199910143411601. N Engl J Med. 1999. PMID: 10519894 Clinical Trial. - Outcome of high-risk stage 3 neuroblastoma with myeloablative therapy and 13-cis-retinoic acid: a report from the Children's Oncology Group.
Park JR, Villablanca JG, London WB, Gerbing RB, Haas-Kogan D, Adkins ES, Attiyeh EF, Maris JM, Seeger RC, Reynolds CP, Matthay KK; Children's Oncology Group. Park JR, et al. Pediatr Blood Cancer. 2009 Jan;52(1):44-50. doi: 10.1002/pbc.21784. Pediatr Blood Cancer. 2009. PMID: 18937318 Free PMC article. Clinical Trial. - Consolidation chemoradiotherapy and autologous bone marrow transplantation versus continued chemotherapy for metastatic neuroblastoma: a report of two concurrent Children's Cancer Group studies.
Stram DO, Matthay KK, O'Leary M, Reynolds CP, Haase GM, Atkinson JB, Brodeur GM, Seeger RC. Stram DO, et al. J Clin Oncol. 1996 Sep;14(9):2417-26. doi: 10.1200/JCO.1996.14.9.2417. J Clin Oncol. 1996. PMID: 8823319 - Retinoic acid post consolidation therapy for high-risk neuroblastoma patients treated with autologous hematopoietic stem cell transplantation.
Peinemann F, van Dalen EC, Tushabe DA, Berthold F. Peinemann F, et al. Cochrane Database Syst Rev. 2015 Jan 29;1:CD010685. doi: 10.1002/14651858.CD010685.pub2. Cochrane Database Syst Rev. 2015. PMID: 25634649 Updated. Review. - High-dose chemotherapy and autologous haematopoietic stem cell rescue for children with high-risk neuroblastoma.
Yalçin B, Kremer LC, Caron HN, van Dalen EC. Yalçin B, et al. Cochrane Database Syst Rev. 2013 Aug 22;(8):CD006301. doi: 10.1002/14651858.CD006301.pub3. Cochrane Database Syst Rev. 2013. PMID: 23970444 Updated. Review.
Cited by
- Epac activation reduces trans-endothelial migration of undifferentiated neuroblastoma cells and cellular differentiation with a CDK inhibitor further enhances Epac effect.
Inuwa RF, Moss D, Quayle J, Swadi R. Inuwa RF, et al. PLoS One. 2024 Nov 4;19(11):e0304547. doi: 10.1371/journal.pone.0304547. eCollection 2024. PLoS One. 2024. PMID: 39495719 Free PMC article. - Systematic Analysis of miR-506-3p Target Genes Identified Key Mediators of Its Differentiation-Inducing Function.
Cardus DF, Smith MT, Vernaza A, Smith JL, Del Buono B, Parajuli A, Lewis EG, Mesa-Diaz N, Du L. Cardus DF, et al. Genes (Basel). 2024 Sep 27;15(10):1268. doi: 10.3390/genes15101268. Genes (Basel). 2024. PMID: 39457392 Free PMC article. - Natural killer cells in neuroblastoma: immunological insights and therapeutic perspectives.
Rados M, Landegger A, Schmutzler L, Rabidou K, Taschner-Mandl S, Fetahu IS. Rados M, et al. Cancer Metastasis Rev. 2024 Sep 18. doi: 10.1007/s10555-024-10212-8. Online ahead of print. Cancer Metastasis Rev. 2024. PMID: 39294470 Review. - Current Knowledge and Perspectives of Immunotherapies for Neuroblastoma.
Mao C, Poimenidou M, Craig BT. Mao C, et al. Cancers (Basel). 2024 Aug 17;16(16):2865. doi: 10.3390/cancers16162865. Cancers (Basel). 2024. PMID: 39199637 Free PMC article. Review. - Inhibition of miR-10b treats metastatic breast cancer by targeting stem cell-like properties.
Halim A, Al-Qadi N, Kenyon E, Conner KN, Mondal SK, Medarova Z, Moore A. Halim A, et al. Oncotarget. 2024 Aug 26;15:591-606. doi: 10.18632/oncotarget.28641. Oncotarget. 2024. PMID: 39189967 Free PMC article.
References
- Matthay KK, Yamashiro D. Neuroblastoma. In: Bast RC, Kufe DW, Pollock RE, et al., editors. Cancer Medicine. London, United Kingdom, B.C.: Decker; 2000. pp. 2185–2197. in. pp.
- Dini G, Philip T, Hartmann O, et al. Bone marrow transplantation for neuroblastoma: A review of 509 cases—EBMT Group. Bone Marrow Transplant. 1989;4(suppl 4):42–46. - PubMed
- Philip T, Zucker JM, Bernard JL, et al. Improved survival at 2 and 5 years in the LMCE1 unselected group of 72 children with stage IV neuroblastoma older than 1 year of age at diagnosis: Is cure possible in a small subgroup? J Clin Oncol. 1991;9:1037–1044. - PubMed
- Stram DO, Matthay KK, O'Leary M, et al. Consolidation chemoradiotherapy and autologous bone marrow transplantation versus continued chemotherapy for metastatic neuroblastoma: A report of two concurrent Children's Cancer Group studies. J Clin Oncol. 1996;14:2417–2426. - PubMed
- Reynolds CP, Kane DJ, Einhorn PA, et al. Response of neuroblastoma to retinoic acid in vitro and in vivo. Prog Clin Biol Res. 1991;366:203–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA081403/CA/NCI NIH HHS/United States
- U10 CA098413/CA/NCI NIH HHS/United States
- CA81403/CA/NCI NIH HHS/United States
- R01 CA082830/CA/NCI NIH HHS/United States
- CA82830/CA/NCI NIH HHS/United States
- CA98413/CA/NCI NIH HHS/United States
- CA13539/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical