Anaphylactic shock depends on endothelial Gq/G11 - PubMed (original) (raw)
Comparative Study
. 2009 Feb 16;206(2):411-20.
doi: 10.1084/jem.20082150. Epub 2009 Jan 26.
Affiliations
- PMID: 19171764
- PMCID: PMC2646572
- DOI: 10.1084/jem.20082150
Comparative Study
Anaphylactic shock depends on endothelial Gq/G11
Hanna Korhonen et al. J Exp Med. 2009.
Abstract
Anaphylactic shock is a severe allergic reaction involving multiple organs including the bronchial and cardiovascular system. Most anaphylactic mediators, like platelet-activating factor (PAF), histamine, and others, act through G protein-coupled receptors, which are linked to the heterotrimeric G proteins G(q)/G(11), G(12)/G(13), and G(i). The role of downstream signaling pathways activated by anaphylactic mediators in defined organs during anaphylactic reactions is largely unknown. Using genetic mouse models that allow for the conditional abrogation of G(q)/G(11)- and G(12)/G(13)-mediated signaling pathways by inducible Cre/loxP-mediated mutagenesis in endothelial cells (ECs), we show that G(q)/G(11)-mediated signaling in ECs is required for the opening of the endothelial barrier and the stimulation of nitric oxide formation by various inflammatory mediators as well as by local anaphylaxis. The systemic effects of anaphylactic mediators like histamine and PAF, but not of bacterial lipopolysaccharide (LPS), are blunted in mice with endothelial G alpha(q)/G alpha(11) deficiency. Mice with endothelium-specific G alpha(q)/G alpha(11) deficiency, but not with G alpha(12)/G alpha(13) deficiency, are protected against the fatal consequences of passive and active systemic anaphylaxis. This identifies endothelial G(q)/G(11)-mediated signaling as a critical mediator of fatal systemic anaphylaxis and, hence, as a potential new target to prevent or treat anaphylactic reactions.
Figures
Figure 1.
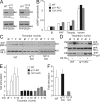
The role of Gq/G11 and G12/G13 in the regulation of NO production and MLC phosphorylation in pulmonary ECs. (A) Lysates of pulmonary ECs prepared from WT, _Gnaq_flox/flox;_Gna11_−/− (q/11-KO), or _Gna12_−/−;_Gna13_flox/flox (12/13-KO) mice were infected with Cre-transducing adenovirus and were analyzed by Western blotting with antibodies directed against Gαq/Gα11, Gα13, or α-tubulin. Arrowheads indicate the position of the 43-kD marker protein. The presented data are representative of at least five experiments performed with samples from different animals. (B) WT Gαq/Gα11-deficient (q/11-KO) and Gα12/Gα13-deficient (12/13-KO) ECs were incubated without and with 1 U/ml thrombin (thromb.), 100 nM PAF, or 100 nM ionomycin (ionom.), and NO bioavailability was assessed in a transfer bioassay by determining cGMP production in detector RFL6 fibroblasts by radioimmunoassay. Shown are the results of three separate experiments (mean values ± SEM). (C–E) WT, Gαq/Gα11- (q/11-KO), and Gα12/Gα13-deficient (12/13-KO) ECs were incubated in the absence or presence of 1 U/ml thrombin for 1, 3, or 10 min, and the amount of phosphorylated MLC (pMLC) was determined using a phosphorylation site-specific antibody (see Materials and methods). Where indicated (Ad-Gαq +), cells had been transfected with Gαq using an adenoviral transfection system. Shown are representative Western blots of cell lysates using the indicated antibodies (C and D) and the results of the densitometric evaluation of three independently performed experiments (E). Shown are mean values ± SEM. Arrowheads indicate the position of the 25- or 43-kD (D, bottom) marker proteins. (F) Effect of 1 U/ml thrombin on RhoA activity in WT, Gαq/Gα11-deficient (q/11-KO), and Gα12/Gα13-deficient lung ECs (12/13-KO). Data are from three independently performed experiments (mean values ± SD).
Figure 2.
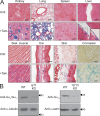
Generation of mice with EC-specific Gαq/Gα11 and Gα12/Gα13 deficiency. (A) Gt(ROSA26)SorCre reporter mice carrying the _tie2_-_Cre_ERT2 transgene were treated with vehicle alone (untr.) or with tamoxifen (+Tam.) and then killed. The indicated organs were sectioned and stained for β-galactosidase activity. Bars, 50 µm. Inserts represent 2× magnifications of the indicated areas. (B) Lysates from lung ECs prepared from tamoxifen-treated WT, EC-Gαq/Gα11-KO (q/11-KO), or EC-Gα12/Gα13-KO (12/13-KO) mice were analyzed by Western blotting with antibodies directed against Gαq/Gα11, Gα13, α-tubulin, or β-actin. Arrows indicate the position of the 43-kD marker protein. Shown are representative data from three independently performed experiments.
Figure 3.
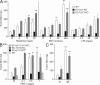
Basal and stimulated endothelial permeability in EC-specific Gαq/Gα11- and Gα12/Gα13-deficient mice. (A and B) Evans blue extravasation was determined in five to eight mice per genotype after intracutaneous injection of 20 µl of the indicated doses of PAF, histamine, LPA (A), or the PAR-1 peptide SFLLRN-NH2 (B). Shown are the amounts of Evans blue determined in skin explants as described in the Materials and methods. (C) At least five mice per genotype were sensitized by intracutaneous injection of anti-DNP IgE antibodies. 24 h later, DNP-HSA was injected i.v., and Evans blue extravasation was determined as described in the Materials and methods. Values are means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared with basal).
Figure 4.
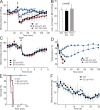
Role of endothelial Gq/G11 and G12/G13 in the systemic effects of histamine, PAF, and LPS. (A) Arterial blood pressure was monitored telemetrically in mice before and after i.v. injection of carrier solution (squares) or 10 mg/kg histamine (circles). Shown are mean values of five to seven animals per genotype ±SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared with WT). The arrow indicates the time point of injection. (B) Arterial blood pressure was monitored telemetrically in anesthesized mice (n ≥ 5 per genotype) before and after i.v. injection of 50 mg/kg l-NAME. Shown is the maximal blood pressure change, in millimeters of mercury, after injection of the NOS inhibitor. Values are the means ± S.D. (C) Arterial blood pressure was monitored telemetrically in mice before and after i.v. injection of 1 mg/kg sodium nitroprusside. Shown are mean values of 5–8 animals per genotype ± S.D. (D and E) Five to six mice per genotype were injected i.v. with 1.9 µg/g PAF, and body temperature (D) and survival (E) were monitored over 120 min. Numbers below the time points of the temperature plot indicate the number of animals still alive at the indicated times (mean values ± SD). (F) Three WT and EC-Gαq/Gα11-KO mice were injected i.p. with 80 µg/g LPS, and the blood pressure was monitored telemetrically for the indicated time period. Shown are the mean values ± SD.
Figure 5.
Passive and active anaphylaxis in endothelium-specific Gαq/Gα11- and Gα12/Gα13-deficient mice. (A and B) Mice were either sensitized with anti-DNP IgE antibodies (A, circles; B, black bars) or received buffer (A, squares; B, white bars). 24 h later, animals were challenged by i.v. injection of DNP-HSA as described in Materials and methods. Shown is the arterial blood pressure monitored telemetrically before and after administration of DNP-HSA (A) as well as the change in hematocrit 10 min after administration of DNP-HSA (B). The data represent mean values of five to six animals per group ±SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared with WT). The arrow in A indicates the time point of DNP-HSA injection. (C and D) Body temperature (C) and survival (D) of mice sensitized with BSA and challenged 14 d later with BSA (circles) or buffer (squares). Experiments were performed with a total of five WT, four EC-Gα12/Gα13-KO, five EC-Gαq/Gα11-KO (immunized), and three EC-Gαq/Gα11-KO (nonimmunized) mice. Numbers below the time points of the temperature plot indicate the number of mice still alive at the indicated times. Shown are mean values ± SD.
Similar articles
- RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock.
Mikelis CM, Simaan M, Ando K, Fukuhara S, Sakurai A, Amornphimoltham P, Masedunskas A, Weigert R, Chavakis T, Adams RH, Offermanns S, Mochizuki N, Zheng Y, Gutkind JS. Mikelis CM, et al. Nat Commun. 2015 Apr 10;6:6725. doi: 10.1038/ncomms7725. Nat Commun. 2015. PMID: 25857352 Free PMC article. - The TNF-like weak inducer of the apoptosis/fibroblast growth factor-inducible molecule 14 axis mediates histamine and platelet-activating factor-induced subcutaneous vascular leakage and anaphylactic shock.
Mendez-Barbero N, Yuste-Montalvo A, Nuñez-Borque E, Jensen BM, Gutiérrez-Muñoz C, Tome-Amat J, Garrido-Arandia M, Díaz-Perales A, Ballesteros-Martinez C, Laguna JJ, Beitia JM, Poulsen LK, Cuesta-Herranz J, Blanco-Colio LM, Esteban V. Mendez-Barbero N, et al. J Allergy Clin Immunol. 2020 Feb;145(2):583-596.e6. doi: 10.1016/j.jaci.2019.09.019. Epub 2019 Oct 31. J Allergy Clin Immunol. 2020. PMID: 31679818 - Sphingosine-1-phosphate receptor 2 protects against anaphylactic shock through suppression of endothelial nitric oxide synthase in mice.
Cui H, Okamoto Y, Yoshioka K, Du W, Takuwa N, Zhang W, Asano M, Shibamoto T, Takuwa Y. Cui H, et al. J Allergy Clin Immunol. 2013 Nov;132(5):1205-1214.e9. doi: 10.1016/j.jaci.2013.07.026. Epub 2013 Sep 8. J Allergy Clin Immunol. 2013. PMID: 24021572 - Mechanisms Governing Anaphylaxis: Inflammatory Cells, Mediators, Endothelial Gap Junctions and Beyond.
Nguyen SMT, Rupprecht CP, Haque A, Pattanaik D, Yusin J, Krishnaswamy G. Nguyen SMT, et al. Int J Mol Sci. 2021 Jul 21;22(15):7785. doi: 10.3390/ijms22157785. Int J Mol Sci. 2021. PMID: 34360549 Free PMC article. Review. - A new G(q)-initiated MAPK signaling pathway in the heart.
Gutkind JS, Offermanns S. Gutkind JS, et al. Dev Cell. 2009 Feb;16(2):163-4. doi: 10.1016/j.devcel.2009.01.021. Dev Cell. 2009. PMID: 19217418 Review.
Cited by
- Sphingosine-1-phosphate receptor 3 promotes leukocyte rolling by mobilizing endothelial P-selectin.
Nussbaum C, Bannenberg S, Keul P, Gräler MH, Gonçalves-de-Albuquerque CF, Korhonen H, von Wnuck Lipinski K, Heusch G, de Castro Faria Neto HC, Rohwedder I, Göthert JR, Prasad VP, Haufe G, Lange-Sperandio B, Offermanns S, Sperandio M, Levkau B. Nussbaum C, et al. Nat Commun. 2015 Apr 2;6:6416. doi: 10.1038/ncomms7416. Nat Commun. 2015. PMID: 25832730 Free PMC article. - Endothelial FAT1 inhibits angiogenesis by controlling YAP/TAZ protein degradation via E3 ligase MIB2.
Li R, Shao J, Jin YJ, Kawase H, Ong YT, Troidl K, Quan Q, Wang L, Bonnavion R, Wietelmann A, Helmbacher F, Potente M, Graumann J, Wettschureck N, Offermanns S. Li R, et al. Nat Commun. 2023 Apr 8;14(1):1980. doi: 10.1038/s41467-023-37671-x. Nat Commun. 2023. PMID: 37031213 Free PMC article. - Glycolysis is integral to histamine-induced endothelial hyperpermeability.
Ziogas A, Sajib MS, Lim JH, Alves TC, Das A, Witt A, Hagag E, Androulaki N, Grossklaus S, Gerlach M, Noll T, Grinenko T, Mirtschink P, Hajishengallis G, Chavakis T, Mikelis CM, Sprott D. Ziogas A, et al. FASEB J. 2021 Mar;35(3):e21425. doi: 10.1096/fj.202001634R. FASEB J. 2021. PMID: 33566443 Free PMC article. - HIF2α signaling inhibits adherens junctional disruption in acute lung injury.
Gong H, Rehman J, Tang H, Wary K, Mittal M, Chaturvedi P, Zhao YY, Komarova YA, Vogel SM, Malik AB. Gong H, et al. J Clin Invest. 2015 Feb;125(2):652-64. doi: 10.1172/JCI77701. Epub 2015 Jan 9. J Clin Invest. 2015. PMID: 25574837 Free PMC article. - Disease associated cellular machinery in anaphylaxis - And the de novo paradigm shift.
Pushparaj PN, Rasool M, Naseer MI, Damiati LA, Kothandaraman N, Gauthaman K, Bhalas S, Manikandan J. Pushparaj PN, et al. Bioinformation. 2015 Jan 30;11(1):43-6. doi: 10.6026/97320630011043. eCollection 2015. Bioinformation. 2015. PMID: 25780280 Free PMC article.
References
- Clark S., Camargo C.A., Jr 2007. Epidemiology of anaphylaxis.Immunol. Allergy Clin. North Am. 27:145–163 - PubMed
- Kemp S.F., Lockey R.F. 2002. Anaphylaxis: a review of causes and mechanisms.J. Allergy Clin. Immunol. 110:341–348 - PubMed
- Neugut A.I., Ghatak A.T., Miller R.L. 2001. Anaphylaxis in the United States: an investigation into its epidemiology.Arch. Intern. Med. 161:15–21 - PubMed
- Sampson H.A., Munoz-Furlong A., Campbell R.L., Adkinson N.F., Jr., Bock S.A., Branum A., Brown S.G., Camargo C.A., Jr., Cydulka R., Galli S.J., et al. 2006. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium.J. Allergy Clin. Immunol. 117:391–397 - PubMed
- Finkelman F.D., Vercelli D. 2007. Anaphylaxis: lessons from mouse models.J. Allergy Clin. Immunol. 120:506–515 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials