C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia - PubMed (original) (raw)
. 2009 Feb 10;106(6):1897-902.
doi: 10.1073/pnas.0805177106. Epub 2009 Jan 26.
Makoto Matsumoto, Osamu Takeuchi, Tetsuhiro Matsuzawa, Eri Ishikawa, Machie Sakuma, Hiroaki Tateno, Jun Uno, Jun Hirabayashi, Yuzuru Mikami, Kiyoshi Takeda, Shizuo Akira, Takashi Saito
Affiliations
- PMID: 19171887
- PMCID: PMC2644135
- DOI: 10.1073/pnas.0805177106
C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia
Sho Yamasaki et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Mincle (also called as Clec4e and Clecsf9) is a C-type lectin receptor expressed in activated phagocytes. Recently, we have demonstrated that Mincle is an FcRgamma-associated activating receptor that senses damaged cells. To search an exogenous ligand(s), we screened pathogenic fungi using cell line expressing Mincle, FcRgamma, and NFAT-GFP reporter. We found that Mincle specifically recognizes the Malassezia species among 50 different fungal species tested. Malassezia is a pathogenic fungus that causes skin diseases, such as tinea versicolor and atopic dermatitis, and fatal sepsis. However, the specific receptor on host cells has not been identified. Mutation of the putative mannose-binding motif within C-type lectin domain of Mincle abrogated Malassezia recognition. Analyses of glycoconjugate microarray revealed that Mincle selectively binds to alpha-mannose but not mannan. Thus, Mincle may recognize specific geometry of alpha-mannosyl residues on Malassezia species and use this to distinguish them from other fungi. Malassezia activated macrophages to produce inflammatory cytokines/chemokines. To elucidate the physiological function of Mincle, Mincle-deficient mice were established. Malassezia-induced cytokine/chemokine production by macrophages from Mincle(-/-) mice was significantly impaired. In vivo inflammatory responses against Malassezia was also impaired in Mincle(-/-) mice. These results indicate that Mincle is the first specific receptor for Malassezia species to be reported and plays a crucial role in immune responses to this fungus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
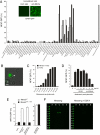
Mincle recognizes Malassezia species and delivers activation signal. (A) Screening of pathogenic fungus for Mincle-ligand activity. Reporter cell line expressing Mincle, FcRγ, and NFAT-GFP was established and left unstimulated or stimulated with plate-coated anti-Mincle mAb (10 μg/ml) for 18 h (inset). Mincle-expressing NFAT-GFP reporter cells were cocultured with indicated pathogenic fungi (open, 1%; closed, 10%) for 18 h, or stimulated with plate-coated anti-Mincle as a positive control (gray). NFAT-GFP induction was analyzed by flow cytometry. Data are representative of 3 independent experiments. (B) Microscopic analysis. Mincle-expressing reporter cells were cocultured with M. pachydermatis (arrowhead) and analyzed with fluorescence microscopy (IX-81; Olympus). (Scale bars: 5 μm.) (C) Dose-dependent activation by M. pachydermatis. Reporter cells expressing Mincle and FcRγ (closed) and only FcRγ (open) were cocultured with indicated amount (cells/ml) of M. Pachydermatis for 18 h. (D) Anti-Mincle mAb blocked _Malassezia_-induced NFAT activation. Reporter cells were cultured with M. pachydermatis in the presence of rat IgG or anti-Mincle mAbs and GFP expression was determined by flow cytometry. (E) Critical role of Mannose-binding motif of Mincle for Malassezia recognition. MincleWT (WT), MincleE169Q/N171D (EPN -> QPD) were expressed together with FcRγ in NFAT-GFP reporter cells. Cells were treated and analyzed as in (D). (F) Mincle specifically recognizes α-mannose. Mincle-Ig (1 μg/ml) precomplexed with Cy3-anti-human IgG (0.5 μg/ml) in TBS containing 1 mM CaCl2 or 10 mM EDTA was applied on the glycoconjugated microarray. After incubation at 20 °C for 3 h, binding was detected by an evanescent-field fluorescence-assisted scanner. Data were analyzed with the Array Pro analyzer ver. 4.5.
Fig. 2.
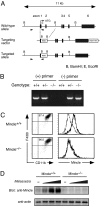
Generation of Mincle-deficient mice. (A) Genomic Mincle structure and targeting constructs with neomycin resistance (Neo) insertion. The Mincle exons are shown as black box. (B) Genomic PCR analysis of Mincle-targeted allele. Genomic DNA isolated from +/+, +/− and −/− mice were amplified with primer pairs for wild-type allele (+) or targeted allele (−) as shown in arrowheads in (A). (C) Surface expression of Mincle. BMMφ from WT (Mincle+/+) and Mincle-deficient (Mincle−/−) mice were stimulated with 1 ng/ml LPS for 18 h and stained with anti-rIgG1-biotin (thin line) or anti-Mincle-biotin (thick line) and Streptavidine-APC. (D) Western blot analysis. Thioglycolate-elicited peritoneal macrophages from Mincle+/+ and Mincle−/− mice were left unstimulated (−) or stimulated with 1, 3, 10 × 106 of M. pachydermatis for 18 h. Cells were lysed and blotted with anti-Mincle and anti-actin mAbs as a control.
Fig. 3.
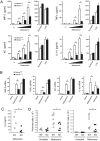
Mincle deficiency failed to respond to Malassezia. (A) _Malassezia_-induced cytokine production. Mincle+/+ and Mincle−/− BMMφ were stimulated with 1, 3, 10 × 106 of M. pachydermatis, 100 μg/ml zymosan or 1 ng/ml LPS for 18 h. *, P < 0.05. (B) _Malassezia_-induced cytokine transcription. BMMφ was stimulated with 10 × 106 M. pachydermatis or 100 μg/ml zymosan for 4 h, and mRNA expression was determined by real time PCR. Relative mRNA levels were expressed as fold induction. *, P < 0.05. (C) Mincle+/− (WT) and Mincle−/− (KO) mice were injected with 4 × 107 M. pachydermatis i.p. After 18 h, the peritoneal cavity was washed out with 5 ml of saline and cytokine concentration was determined by ELISA. Each symbol represents an individual mouse. Data are representative of two independent experiments. *, P < 0.05. (D) Mincle+/+ or Mincle+/− (WT) and Mincle−/− (KO) mice were injected with 2.5 × 107 M. pachydermatis i.p. At 20 h after injection, peritoneal cells were stained with CD11b and Gr1 and analyzed by flowcytometry. Total number of peritoneal cells (Left) and CD11b+Gr1+ cells (Right) were indicated. Data are representative of two independent experiments. *, P < 0.05.
Similar articles
- Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia.
Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, Saito T, Okawa Y, Shibata N, Miyamoto T, Yamasaki S. Ishikawa T, et al. Cell Host Microbe. 2013 Apr 17;13(4):477-88. doi: 10.1016/j.chom.2013.03.008. Cell Host Microbe. 2013. PMID: 23601109 - Intracellular metabolite β-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity.
Nagata M, Izumi Y, Ishikawa E, Kiyotake R, Doi R, Iwai S, Omahdi Z, Yamaji T, Miyamoto T, Bamba T, Yamasaki S. Nagata M, et al. Proc Natl Acad Sci U S A. 2017 Apr 18;114(16):E3285-E3294. doi: 10.1073/pnas.1618133114. Epub 2017 Apr 3. Proc Natl Acad Sci U S A. 2017. PMID: 28373578 Free PMC article. - Mincle is an ITAM-coupled activating receptor that senses damaged cells.
Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Yamasaki S, et al. Nat Immunol. 2008 Oct;9(10):1179-88. doi: 10.1038/ni.1651. Epub 2008 Sep 7. Nat Immunol. 2008. PMID: 18776906 - Immune Recognition of Pathogen-Derived Glycolipids Through Mincle.
Miyake Y, Yamasaki S. Miyake Y, et al. Adv Exp Med Biol. 2020;1204:31-56. doi: 10.1007/978-981-15-1580-4_2. Adv Exp Med Biol. 2020. PMID: 32152942 Review. - Mincle: 20 years of a versatile sensor of insults.
Lu X, Nagata M, Yamasaki S. Lu X, et al. Int Immunol. 2018 May 24;30(6):233-239. doi: 10.1093/intimm/dxy028. Int Immunol. 2018. PMID: 29726997 Review.
Cited by
- The adaptor protein CARD9, from fungal immunity to tumorigenesis.
Wang Y, Zhang D, Hou Y, Shen S, Wang T. Wang Y, et al. Am J Cancer Res. 2020 Aug 1;10(8):2203-2225. eCollection 2020. Am J Cancer Res. 2020. PMID: 32905547 Free PMC article. Review. - C-type lectin Mincle mediates cell death-triggered inflammation in acute kidney injury.
Tanaka M, Saka-Tanaka M, Ochi K, Fujieda K, Sugiura Y, Miyamoto T, Kohda H, Ito A, Miyazawa T, Matsumoto A, Aoe S, Miyamoto Y, Tsuboi N, Maruyama S, Suematsu M, Yamasaki S, Ogawa Y, Suganami T. Tanaka M, et al. J Exp Med. 2020 Nov 2;217(11):e20192230. doi: 10.1084/jem.20192230. J Exp Med. 2020. PMID: 32797195 Free PMC article. - A20 and ABIN-3 possibly promote regression of trehalose 6,6'-dimycolate (TDM)-induced granuloma by interacting with an NF-kappa B signaling protein, TAK-1.
Sakai Y, Uchida K, Nakayama H. Sakai Y, et al. Inflamm Res. 2012 Mar;61(3):245-53. doi: 10.1007/s00011-011-0406-6. Epub 2011 Dec 16. Inflamm Res. 2012. PMID: 22173278 - Expression and function of macrophage-inducible C-type lectin (Mincle) in inflammation driven parturition in fetal membranes and myometrium.
Lim R, Lappas M. Lim R, et al. Clin Exp Immunol. 2019 Jul;197(1):95-110. doi: 10.1111/cei.13281. Epub 2019 Mar 13. Clin Exp Immunol. 2019. PMID: 30793298 Free PMC article. - Dectin-2 Is a C-Type Lectin Receptor that Recognizes Pneumocystis and Participates in Innate Immune Responses.
Kottom TJ, Hebrink DM, Jenson PE, Marsolek PL, Wüthrich M, Wang H, Klein B, Yamasaki S, Limper AH. Kottom TJ, et al. Am J Respir Cell Mol Biol. 2018 Feb;58(2):232-240. doi: 10.1165/rcmb.2016-0335OC. Am J Respir Cell Mol Biol. 2018. PMID: 28886250 Free PMC article.
References
- Matsumoto M, et al. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol. 1999;163:5039–5048. - PubMed
- Yamasaki S, et al. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. - PubMed
- Flornes LM, et al. Identification of lectin-like receptors expressed by antigen presenting cells and neutrophils and their mapping to a novel gene complex. Immunogenetics. 2004;56:506–517. - PubMed
- Saijo S, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. - PubMed
- Sato K, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J Biol Chem. 2006;281:38854–38866. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials