Endogenous endothelial cell signaling systems maintain vascular stability - PubMed (original) (raw)
Review
Endogenous endothelial cell signaling systems maintain vascular stability
Nyall R London et al. Angiogenesis. 2009.
Abstract
The function of the endothelium is to provide a network to allow delivery of oxygen and nutrients to tissues throughout the body. This network comprises adjacent endothelial cells that utilize adherens junction proteins such as vascular endothelial cadherin (VE-cadherin) to maintain the appropriate level of vascular permeability. The disruption of VE-cadherin interactions during pathologic settings can lead to excessive vascular leak with adverse effects. Endogenous cell signaling systems have been defined, which help to maintain the proper level of vascular stability. Perhaps the best described system is Angiopoietin-1 (Ang-1). Ang-1 acting through its receptor Tie2 generates a well-described set of signaling events ultimately leading to enhanced vascular stability. In this review, we will focus on what is known about additional endogenous cell signaling systems that stabilize the vasculature, and using Ang-1/Tie2 as a model, we will address where our understanding of these additional systems is lacking.
Figures
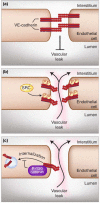
Figure 1. VE-cadherin interactions are disrupted by phosphorylation and endocytosis
(a) Under normal conditions, VE-cadherin forms tight interactions that inhibit excessive vascular leak. (b) Kinases such as Src can phosphorylate the cytoplasmic tail of VE-cadherin, resulting in the disruption of VE-cadherin interactions and increased vascular permeability. (c) VE-cadherin interactions are also disrupted through endocytosis, resulting in increased vascular leak. The binding of p-120 catenin to the cytoplasmic tail of VE-cadherin inhibits internalization resulting in enhanced vascular stability.
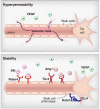
Figure 2. Endogenous cell signaling systems decrease vascular hyperpermeability
(top) VEGF disrupts the barrier function of the endothelium resulting in enhanced vascular leak. (bottom) Cell signaling systems such as Slit-Robo4, Ang-1-Tie2, and Dll4-Notch stabilize the vasculature.
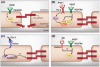
Figure 3. Endogenous cell signaling systems enhance vascular stability
(a) VEGF disrupts VE-cadherin interactions through activation of Src resulting in the phosphorylation of the cytoplasmic tail of VE-cadherin. (b) Ang-1 through its receptor Tie2 activates Rho. This causes the activation of mDia, which sequesters Src resulting in the inhibition of Src and stabilization of VE-cadherin. (c) Dll4 binds Notch resulting in the cleavage of the NICD and its transfer to the nucleus. The resulting signaling events that affect endothelial cell junctional stability are unknown. (d) Slit through a Robo4 dependent mechanism inhibits VEGF-induced activation of Src. The signaling events from Robo4 to Src are unknown.
Figure 4. The CCM genes define an intracellular protein complex promoting vascular stability
The three genes associated with cerebral cavernous malformations (KRIT1, CCM2, and PDCD10) interact with each other and a variety of other intracellular and cell surface proteins to enhance the stability of endothelial cell junctions and the cellular cytoskeleton. As described in the text, such interactions include the Heart of glass (HEG) cell surface receptor, ß-catenin (ß-cat), small GTPases including RAP1 and Rho family GTPases, and proteins of MAP kinase signaling cascades.
Similar articles
- Angiopoietin-2-induced blood-brain barrier compromise and increased stroke size are rescued by VE-PTP-dependent restoration of Tie2 signaling.
Gurnik S, Devraj K, Macas J, Yamaji M, Starke J, Scholz A, Sommer K, Di Tacchio M, Vutukuri R, Beck H, Mittelbronn M, Foerch C, Pfeilschifter W, Liebner S, Peters KG, Plate KH, Reiss Y. Gurnik S, et al. Acta Neuropathol. 2016 May;131(5):753-73. doi: 10.1007/s00401-016-1551-3. Epub 2016 Mar 1. Acta Neuropathol. 2016. PMID: 26932603 Free PMC article. - Gαs Relays Sphingosine-1-Phosphate Receptor 1 Signaling to Stabilize Vascular Endothelial-Cadherin at Endothelial Junctions to Control Mouse Embryonic Vascular Integrity.
Shao X, Liu K, Fan Y, Ding Z, Chen M, Zhu M, Weinstein LS, Li H, Li H. Shao X, et al. J Genet Genomics. 2015 Nov 20;42(11):613-624. doi: 10.1016/j.jgg.2015.08.006. Epub 2015 Oct 20. J Genet Genomics. 2015. PMID: 26674379 - Regulation of vascular endothelial junction stability and remodeling through Rap1-Rasip1 signaling.
Wilson CW, Ye W. Wilson CW, et al. Cell Adh Migr. 2014;8(2):76-83. doi: 10.4161/cam.28115. Cell Adh Migr. 2014. PMID: 24622510 Free PMC article. - Angiopoietin-1/Tie2 receptor signaling in vascular quiescence and angiogenesis.
Fukuhara S, Sako K, Noda K, Zhang J, Minami M, Mochizuki N. Fukuhara S, et al. Histol Histopathol. 2010 Mar;25(3):387-96. doi: 10.14670/HH-25.387. Histol Histopathol. 2010. PMID: 20054809 Review. - The role of the Angiopoietins in vascular morphogenesis.
Thomas M, Augustin HG. Thomas M, et al. Angiogenesis. 2009;12(2):125-37. doi: 10.1007/s10456-009-9147-3. Epub 2009 May 16. Angiogenesis. 2009. PMID: 19449109 Review.
Cited by
- Barrier stabilizing mediators in regulation of microvascular endothelial permeability.
Huang QB. Huang QB. Chin J Traumatol. 2012;15(2):105-12. doi: 10.3760/cma.j.issn.1008-1275.2012.02.008. Chin J Traumatol. 2012. PMID: 22480675 Free PMC article. Review. - Serum response factor is required for cell contact maintenance but dispensable for proliferation in visceral yolk sac endothelium.
Holtz ML, Misra RP. Holtz ML, et al. BMC Dev Biol. 2011 Mar 14;11:18. doi: 10.1186/1471-213X-11-18. BMC Dev Biol. 2011. PMID: 21401944 Free PMC article. - VEGFR2 and Src kinase inhibitors suppress Andes virus-induced endothelial cell permeability.
Gorbunova EE, Gavrilovskaya IN, Pepini T, Mackow ER. Gorbunova EE, et al. J Virol. 2011 Mar;85(5):2296-303. doi: 10.1128/JVI.02319-10. Epub 2010 Dec 22. J Virol. 2011. PMID: 21177802 Free PMC article. - Protein therapeutics for cardiac regeneration after myocardial infarction.
Segers VF, Lee RT. Segers VF, et al. J Cardiovasc Transl Res. 2010 Oct;3(5):469-77. doi: 10.1007/s12265-010-9207-5. Epub 2010 Jul 7. J Cardiovasc Transl Res. 2010. PMID: 20607468 Free PMC article. Review. - Ferulic Acid Protects Endothelial Cells from Hypoxia-Induced Injury by Regulating MicroRNA-92a.
Huang Y, Tian L, Liu Y, Liu J, Huang J. Huang Y, et al. Appl Bionics Biomech. 2022 Jul 31;2022:6148361. doi: 10.1155/2022/6148361. eCollection 2022. Appl Bionics Biomech. 2022. PMID: 35959508 Free PMC article. Retracted.
References
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. - PubMed
- Vestweber D, Winderlich M, Cagna G, Nottebaum AF. Cell adhesion dynamics at endothelial junctions: VE-cadherin as a major player. Trends Cell Biol. 2008 - PubMed
- Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. - PubMed
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. - PubMed
- Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5:1359–1364. - PubMed
Publication types
MeSH terms
Grants and funding
- K08 HL079095/HL/NHLBI NIH HHS/United States
- R01 HL068873/HL/NHLBI NIH HHS/United States
- R01 HL077671/HL/NHLBI NIH HHS/United States
- R01 HL084516/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous