Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells - PubMed (original) (raw)
Neuropilin-1/GIPC1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells
Donatella Valdembri et al. PLoS Biol. 2009.
Expression of concern in
- Expression of Concern: Neuropilin-1/GIPC1 Signaling Regulates α5β1 Integrin Traffic and Function in Endothelial Cells.
PLOS Biology Editors. PLOS Biology Editors. PLoS Biol. 2022 Oct 5;20(10):e3001840. doi: 10.1371/journal.pbio.3001840. eCollection 2022 Oct. PLoS Biol. 2022. PMID: 36198142 Free PMC article. No abstract available.
Abstract
Neuropilin 1 (Nrp1) is a coreceptor for vascular endothelial growth factor A165 (VEGF-A165, VEGF-A164 in mice) and semaphorin 3A (SEMA3A). Nevertheless, Nrp1 null embryos display vascular defects that differ from those of mice lacking either VEGF-A164 or Sema3A proteins. Furthermore, it has been recently reported that Nrp1 is required for endothelial cell (EC) response to both VEGF-A165 and VEGF-A121 isoforms, the latter being incapable of binding Nrp1 on the EC surface. Taken together, these data suggest that the vascular phenotype caused by the loss of Nrp1 could be due to a VEGF-A164/SEMA3A-independent function of Nrp1 in ECs, such as adhesion to the extracellular matrix. By using RNA interference and rescue with wild-type and mutant constructs, we show here that Nrp1 through its cytoplasmic SEA motif and independently of VEGF-A165 and SEMA3A specifically promotes alpha5beta1-integrin-mediated EC adhesion to fibronectin that is crucial for vascular development. We provide evidence that Nrp1, while not directly mediating cell spreading on fibronectin, interacts with alpha5beta1 at adhesion sites. Binding of the homomultimeric endocytic adaptor GAIP interacting protein C terminus, member 1 (GIPC1), to the SEA motif of Nrp1 selectively stimulates the internalization of active alpha5beta1 in Rab5-positive early endosomes. Accordingly, GIPC1, which also interacts with alpha5beta1, and the associated motor myosin VI (Myo6) support active alpha5beta1 endocytosis and EC adhesion to fibronectin. In conclusion, we propose that Nrp1, in addition to and independently of its role as coreceptor for VEGF-A165 and SEMA3A, stimulates through its cytoplasmic domain the spreading of ECs on fibronectin by increasing the Rab5/GIPC1/Myo6-dependent internalization of active alpha5beta1. Nrp1 modulation of alpha5beta1 integrin function can play a causal role in the generation of angiogenesis defects observed in Nrp1 null mice.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures
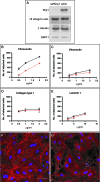
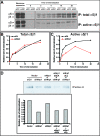
Figure 1. Nrp1 Is Required for EC Adhesion to FN and FN Fibrillogenesis
(A) Western blot analysis of protein expression in human ECs silenced for Nrp1 (si_h_Nrp1) or transfected with control siRNA (siCtl). (B–E) Comparison between siCtl (black lines) and si_h_Nrp1 (red lines) transfected ECs adhering to different ECM proteins, i.e., FN (B), VT (C), COLL-I (D), and LN (E). (F–G) Confocal scanning microscopy analysis of endogenous FN fibrils in siCtl (F) or si_h_Nrp1 (G) transfected ECs. DAPI was used to stain nuclei. White bar in (F) corresponds to 50 μm.
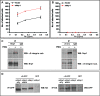
Figure 2. Nrp1 Regulates EC Adhesion to FN and FN Fibrillogenesis via Its Cytoplasmic Domain
(A) Schematic representation of _m_Nrp1 full-length and deletion mutants. (B) Western blot analysis of endogenous _h_Nrp1, exogenously transduced _m_Nrp1 constructs, and endogenous β-tubulin expression in ECs transfected with siCtl or si_h_Nrp1 and afterward transduced with PINCO retrovirus carrying _m_Nrp1 constructs. Western blot analysis of biotinylated _m_Nrp1 constructs reveals their correct expression on the surface of ECs. (C) Comparison of wild-type full-length _m_Nrp1 (_m_Nrp1) and _m_Nrp1dSEA and _m_Nrp1dCy efficiency in rescuing the defective adhesion of si_h_Nrp1 ECs to FN. (D–F) Impairment of FN fibrillogenesis in si_h_Nrp1 ECs is rescued by _m_Nrp1 (D) but not by _m_Nrp1dSEA (E) and _m_Nrp1dCy constructs (F). DAPI was used to stain nuclei. White bar in (D) corresponds to 50 μm. (G) _m_Nrp1 overexpression stimulates NIH 3T3 fibroblast adhesion to FN, whereas neither _m_Nrp1dSEA nor _m_Nrp1dCy is active in this respect. (H,I) Silencing _h_Nrp1 completely blocks VEGF-A165-dependent stimulation (H) and SEMA3A-dependent inhibition (I) of human EC adhesion to FN. (J) Silencing _h_Nrp1 in ECs does not impair the _h_Nrp2-dependent inhibition of cell adhesion to FN by SEMA3F.
Figure 3. Nrp1 Regulation of Cell Adhesion to FN Requires α5β1 Integrin
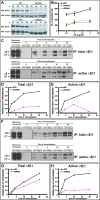
(A,B) CHO cells expressing (CHO B2α27) (A) or lacking (CHO B2) (B) the α5 integrin subunit were transfected with _m_Nrp1 and allowed to adhere on FN. Overexpression of _m_Nrp1 stimulated CHO cell adhesion to FN in the presence but not in the absence of α5β1 integrin. (C) Immunoprecipitation of endogenous _h_Nrp1 and α5β1 integrin from ECs preincubated for 10 min either in the absence or in the presence of 0.6 μM PMQ, followed, respectively, by Western blotting with anti-α5-integrin Ab and anti-Nrp1 Ab. In ECs, Nrp1 associates with α5β1 integrin, and the recycling inhibitor PMQ increases the stoichiometry of their interaction. (D) NIH 3T3 fibroblasts were cotransfected with the GFP-tagged α5 integrin subunit or GFP alone together with an HA-tagged version of _m_Nrp1 full-length (_m_Nrp1) or _m_Nrp1dSEA or _m_Nrp1dCy deletion constructs. α5-GFP, but not GFP alone, coimmunoprecipitates with all HA-tagged _m_Nrp1 constructs.
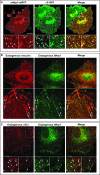
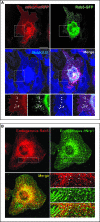
Figure 4. Nrp1 Colocalizes with α5β1 Integrin at Adhesion Sites and Trafficking Vesicles
Fluorescent confocal microscopy analysis of untransfected or transfected ECs allowed to adhere for 3 h on FN. (A) In transfected ECs, _m_Nrp1-mRFP (red) and α5-GFP (green) are in close association at adhesion sites (arrows) and colocalize in intracellular vesicles (arrowheads), as visible in merging (right panels). (B) Immunofluorescence analysis reveals that both endogenous _h_Nrp1 and vinculin are enriched in adhesion sites of human ECs (arrows). (C) Similar to what is observed with fluorescent protein-tagged constructs, immunofluorescence analysis showed that endogenous _h_Nrp1 and α5β1 integrin closely associate in adhesion sites (arrows) and colocalize in intracellular vesicles (arrowheads). Lower panels are magnifications of the indicated boxed areas. White bar in (A) corresponds to 25 μm.
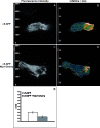
Figure 5. TIRF/FLIM Analysis of the Fluorescence Resonance Energy Transfer between α5-GFP and _m_Nrp1-Cherry
NIH 3T3 fibroblasts were transfected with either α5-GFP alone (A,B) or cotransfected with α5-GFP and _m_Nrp1-Cherry (C,D) and plated onto FN-coated glass-bottom dishes. (A,C) Fluorescent intensity images of α5-GFP excited in TIRF with a 473-nm laser show the expected α5-GFP localization in adhesion sites. (B,D) Pseudocolor images of the spatial distribution of donor (α5-GFP) fluorescence lifetimes τ (measured in nanoseconds) were obtained by frequency-domain FLIM analysis of TIRF fluorescence images shown in (A) and (C). (E) In comparison with cells transfected with α5-GFP alone (n = 7), the donor lifetime τ is decreased (from 2.6 ± 0.05 to 2.3 ± 0.06 ns) in adhesion sites of cells expressing both α5-GFP and _m_Nrp1-Cherry (n = 7; P = 0.00000005).
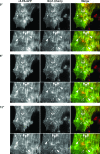
Figure 6. Nrp1 Regulates the Traffic of Active α5β1 Integrin in ECs
(A) Time-course assays reveal an impairment of active but not total α5β1 integrin internalization in ECs silenced for _h_Nrp1 (si_h_N1) in comparison with cells transfected with control siRNA (siCtl). (B,C) Relative quantifications of time-course internalization assays shown in (A) of total (B) and active (C) α5β1 integrin are depicted. (D) Wild-type _m_Nrp1, but neither _m_Nrp1dSEA nor _m_Nrp1dCy deletion constructs, was able to rescue the early (4 min) internalization defects of active α5β1 integrin in si_h_Nrp1 ECs as quantified in the lower histogram.
Figure 7. Rapid Turnover of α5β1 Integrin from Adhesion Sites into Nrp1-Positive Vesicles
A representative NIH 3T3 cell was cotransfected with _m_Nrp1-Cherry and α5-PA-GFP, photoactivated in TIRF, and observed in time-lapse epifluorescence microscopy. After photoactivation, α5-PA-GFP integrin (left panels) was found in elongated adhesive structures (i.e., fibrillar adhesions; arrows), where it colocalized with _m_Nrp1-Cherry (middle panels), as evident in merged images as well (right panels). α5-PA-GFP integrin is also present in nascent vesicles near adhesive structures (arrowheads). Over time GFP fluorescence intensity (left panels) is constant in some vesicles (solid arrowheads), while it increases in others (empty arrowheads), that become progressively enriched in α5-PA-GFP integrin deriving from membrane adhesion sites. _m_Nrp1-Cherry (middle panels) is also present in the same vesicles (solid and empty arrowheads), as visible in merged images (right panels). At each time point, lower panels are magnifications of the corresponding upper panels (see also Video S1).
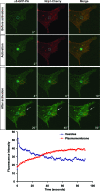
Figure 8. Upon Photoactivation in _m_Nrp1-Cherry-Positive Vesicles, α5-PA-GFP Recycles Back to Membrane Adhesions
NIH 3T3 cells were cotransfected with _m_Nrp1-Cherry and α5-PA-GFP. α5-PA-GFP was then locally photoactivated in vesicles containing _m_Nrp1-Cherry (blue circle) and followed by time-lapse confocal microscopy. Fluorescence intensity was measured over time in Nrp1-positive vesicles (blue circle) and at the plasma membrane (red circle). The time-lapse plot (lower panel) shows that the α5-PA-GFP fluorescence intensity decreases in the vesicles, while it increases at the plasma membrane. This means that α5-PA-GFP integrin is recycled from Nrp1-containing vesicles to the plasma membrane, where it appears to be enriched in what looks like adhesive structures (red circle and white arrows) (see also Video S3).
Figure 9. Nrp1 and Active α5β1 Integrin Localize into Rab5-Positive Early Endosomes
(A) Fluorescent confocal microscopy analysis of ECs transfected with _m_Nrp1-mRFP and Rab5-GFP and then incubated with the anti-active-α5β1 mAb (SNAKA51). _m_Nrp1-mRFP and active α5β1 colocalize into Rab5-GFP-positive early endosomes as shown in merging (arrowheads). Lower panels are magnifications of the boxed areas shown in the upper panels. (B) Immunofluorescent confocal microscopy analysis of endogenous _h_Nrp1 and Rab5 localization in ECs. As described previously, _h_Nrp1 is concentrated in elongated adhesion sites and in vesicular structures. Endogenous Rab5 colocalizes with _h_Nrp1 into early endosomes (solid and empty arrowheads), many of which are located near adhesion sites (empty arrowheads). Lower-right panels are magnifications of the boxed areas shown in the other panels. White bar in (A) corresponds to 25 μm.
Figure 10. In ECs GIPC1 and Myo6 Regulate α5β1 Integrin Traffic and Function
(A) Western blot analysis of protein expression in ECs silenced for human GIPC1 (si_h_GIPC1) or Myo6 (si_h_Myo6) or transfected with control siRNA (siCtl) reveals an efficient silencing of GIPC1 or Myo6 at 96 h after the second oligofection. (B) Comparison between siCtl (black) and either si_h_GIPC1 (red) or si_h_Myo6 (green) transfected ECs adhering to FN. (C) Time-course analysis reveals an impairment of both total and active α5β1 integrin internalization in ECs silenced for _h_GIPC1 in comparison with control cells (siCtl). (D,E) Relative quantification of time-lapse endocytosis assay (shown in (C)) of total (D) or active (E) α5β1 integrin in ECs silenced for _h_GIPC1. (F) Time-course analysis reveals a significant impairment of active but not total α5β1 integrin internalization in ECs silenced for _h_Myo6 in comparison with control cells (siCtl). (G,H) Relative quantification of time-course endocytosis assay (shown in (F)) of total (G) or active (H) α5β1 integrin in ECs silenced for _h_Myo6.
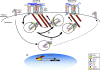
Figure 11. Model for Nrp1 Regulation of α5β1 Integrin Traffic and Function in ECs
(A) At adhesive sites of ECs spreading on FN, Nrp1, via its cytoplasmic association with oligomers of the endocytic adaptor GIPC1, promotes the Rab5/Rab21-dependent internalization of active α5β1 integrin. Once endocytosed, active α5β1 is then recycled back from Nrp1-positive vesicles to the cell surface, thus favoring the dynamic rehandling of newly forming adhesion sites. GIPC1 oligomers could facilitate the association of the α5 integrin subunit with the Bin-Amphiphysin-Rvs (BAR) protein and Rab5/Rab21 interactor APPL1. Myo6 associates with and assists GIPC1 in promoting active α5β1 endocytosis and the ensuing postendocytic traffic. (B) Moreover, in adherent cells a steady endo-exocytic flow of (active) α5β1 integrins from and toward existing ECM adhesions could allow cells to rapidly adjust polarity and cell–ECM contacts in response to extracellular stimuli. (C) In addition, Myo6 can translocate to the EC nucleus, where it stimulates the RNA-polymerase-II-dependent transcription of the FN1 gene.
Similar articles
- Imatinib inhibits VEGF-independent angiogenesis by targeting neuropilin 1-dependent ABL1 activation in endothelial cells.
Raimondi C, Fantin A, Lampropoulou A, Denti L, Chikh A, Ruhrberg C. Raimondi C, et al. J Exp Med. 2014 Jun 2;211(6):1167-83. doi: 10.1084/jem.20132330. Epub 2014 May 26. J Exp Med. 2014. PMID: 24863063 Free PMC article. - The cytoplasmic domain of neuropilin-1 regulates focal adhesion turnover.
Seerapu HR, Borthakur S, Kong N, Agrawal S, Drazba J, Vasanji A, Fantin A, Ruhrberg C, Buck M, Horowitz A. Seerapu HR, et al. FEBS Lett. 2013 Nov 1;587(21):3392-9. doi: 10.1016/j.febslet.2013.08.040. Epub 2013 Sep 8. FEBS Lett. 2013. PMID: 24021649 Free PMC article. - VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation.
Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC. Herzog B, et al. Mol Biol Cell. 2011 Aug 1;22(15):2766-76. doi: 10.1091/mbc.E09-12-1061. Epub 2011 Jun 8. Mol Biol Cell. 2011. PMID: 21653826 Free PMC article. - Neuropilin-1 enforces extracellular matrix signalling via ABL1 to promote angiogenesis.
Raimondi C. Raimondi C. Biochem Soc Trans. 2014 Oct;42(5):1429-34. doi: 10.1042/BST20140141. Biochem Soc Trans. 2014. PMID: 25233427 Review. - Neuropilin regulation of angiogenesis.
Lampropoulou A, Ruhrberg C. Lampropoulou A, et al. Biochem Soc Trans. 2014 Dec;42(6):1623-8. doi: 10.1042/BST20140244. Biochem Soc Trans. 2014. PMID: 25399580 Review.
Cited by
- Neuropilins and liver.
Elpek GÖ. Elpek GÖ. World J Gastroenterol. 2015 Jun 21;21(23):7065-73. doi: 10.3748/wjg.v21.i23.7065. World J Gastroenterol. 2015. PMID: 26109793 Free PMC article. Review. - Neuropilin-2 regulates α6β1 integrin in the formation of focal adhesions and signaling.
Goel HL, Pursell B, Standley C, Fogarty K, Mercurio AM. Goel HL, et al. J Cell Sci. 2012 Jan 15;125(Pt 2):497-506. doi: 10.1242/jcs.094433. Epub 2012 Feb 2. J Cell Sci. 2012. PMID: 22302985 Free PMC article. - Neuropilin-1 peptide-like ligands with proline mimetics, tested using the improved chemiluminescence affinity detection method.
Puszko AK, Sosnowski P, Tymecka D, Raynaud F, Hermine O, Lepelletier Y, Misicka A. Puszko AK, et al. Medchemcomm. 2019 Jan 25;10(2):332-340. doi: 10.1039/c8md00537k. eCollection 2019 Feb 1. Medchemcomm. 2019. PMID: 30881620 Free PMC article. - VEGF targets the tumour cell.
Goel HL, Mercurio AM. Goel HL, et al. Nat Rev Cancer. 2013 Dec;13(12):871-82. doi: 10.1038/nrc3627. Nat Rev Cancer. 2013. PMID: 24263190 Free PMC article. Review. - Neuropilins in the Context of Tumor Vasculature.
Niland S, Eble JA. Niland S, et al. Int J Mol Sci. 2019 Feb 1;20(3):639. doi: 10.3390/ijms20030639. Int J Mol Sci. 2019. PMID: 30717262 Free PMC article. Review.
References
- Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–658. - PubMed
- Hynes RO, Zhao Q. The evolution of cell adhesion. J Cell Biol. 2000;150:F89–F96. - PubMed
- Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost. 2007;5(Suppl 1):32–40. - PubMed
- Risau W, Lemmon V. Changes in the vascular extracellular matrix during embryonic vasculogenesis and angiogenesis. Dev Biol. 1988;125:441–450. - PubMed
- Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous