Steroids initiate a signaling cascade that triggers rapid sporulation in Dictyostelium - PubMed (original) (raw)
Steroids initiate a signaling cascade that triggers rapid sporulation in Dictyostelium
Christophe Anjard et al. Development. 2009 Mar.
Abstract
Encapsulation of prespore cells of Dictyostelium discoideum is controlled by several intercellular signals to ensure appropriate timing during fruiting body formation. Acyl-CoA-binding protein, AcbA, is secreted by prespore cells and processed by the prestalk protease TagC to form the 34 amino acid peptide SDF-2 that triggers rapid encapsulation. AcbA is secreted when gamma-aminobutyric acid (GABA) is released from prespore cells and binds to GrlE, a G protein-coupled receptor (GPCR). Analysis of SDF-2 production in mutant strains lacking Galpha subunits and GPCRs, either as pure populations or when mixed with other mutant strains, uncovered the non-cell-autonomous roles of GrlA, Galpha4 and Galpha7. We found that Galpha7 is essential for the response to GABA and is likely to be coupled to GrlE. GrlA-null and Galpha4-null cells respond normally to GABA but fail to secrete it. We found that they are necessary for the response to a small hydrophobic molecule, SDF-3, which is released late in culmination. Pharmacological inhibition of steroidogenesis during development blocked the production of SDF-3. Moreover, the response to SDF-3 could be blocked by the steroid antagonist mifepristone, whereas hydrocortisone and other steroids mimicked the effects of SDF-3 when added in the nanomolar range. It appears that SDF-3 is a steroid that elicits rapid release of GABA by acting through the GPCR GrlA, coupled to G protein containing the Galpha4 subunit. SDF-3 is at the head of the cascade that amplifies the signal for encapsulation to ensure the rapid, synchronous formation of spores.
Figures
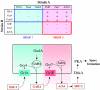
Fig. 1.
Synergy between Dictyostelium discoideum strains unable to produce SDF-2. (Above) Mutant strains lacking one of six proteins essential for production of SDF-2 were mixed in equal proportions with cells lacking another of the indicated proteins. After developing together for 24 hours, fruiting bodies were collected in cAMP buffer and the supernatant tested for SDF-2 activity. Minus indicates that less than 0.2 units of SDF-2/103 cells were recovered, whereas plus indicates that more than 5000 units of SDF-2/103 cells were produced. Strains that failed to synergize to produce SDF-2 constituted a synergy group. (Below) A signaling cascade is proposed to connect the two groups. GrlA and GrlE are GPCRs and GadA is glutamate decarboxylase. Gα4 and Gα7 are trimeric G protein components. AcbA, acyl-CoA-binding protein. GABA, γ-aminobutyric acid. SDF-2 is a 34 amino acid signaling peptide.
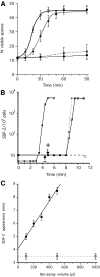
Fig. 2.
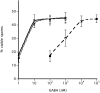
Time course of spore formation and SDF-2 production after induction by SDF-3. (A) Induction of spore formation by SDF-2 or SDF-3. KP cells were plated in 24-well plates at 1×103 cells/cm2 in cAMP buffer. After overnight incubation at 22°C, 10 pM SDF-2 or 10 units SDF-3 were added and the number of spores was scored every 10 minutes for 1 hour and at 90 minutes. Antibodies against GABA or AcbA were added prior to induction. Open circle, induction by SDF-2; black diamond, induction by SDF-3; open diamond, induction by SDF-3 in the presence of anti-AcbA antibodies (1/500); black circle, induction by SDF-3 in presence of anti-GABA antibodies (1/5000). Each experiment was repeated at least three times. (B) Induction of SDF-2 production by SDF-3, hydrocortisone or GABA. 10 nM GABA (triangle), 10 nM hydrocortisone (diamond) or 10 units SDF-3 (square) were added to KP cells and aliquots were taken at the indicated times. SDF-2 production was measured after purification over anion-exchange resin. In another set of experiments, anti-GABA antibodies were added just prior to SDF-3 (circle) or at the indicated time after SDF-3 addition (arrows). In these experiments, SDF-2 production was measured 11 minutes after SDF-3 addition. The arrow with a minus sign indicates the time when addition of anti-GABA antibodies prevented SDF-2 production, and the arrow with a plus sign indicates the time when addition of anti-GABA antibodies did not prevent SDF-2 production. The data for GABA are derived from Anjard and Loomis (Anjard and Loomis, 2005). (C) The delay between SDF-3 addition and SDF-2 production increases with the volume of the bioassay. KP cells were starved overnight in 500 μl cAMP buffer (standard conditions). Just before being used in the assay the volume was adjusted to that indicated, by adding or removing cAMP buffer. SDF-2 production was stimulated by addition of 2 units SDF-3/100 μl (black square) or 10 nM GABA (open square). Aliquots were taken every 30 seconds and tested for SDF-2 production. The earliest time SDF-2 could be detected for a given volume was plotted. Error bars corresponding to ±15 seconds are given. Each experiment was repeated two to three times.
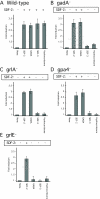
Fig. 3.
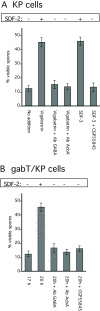
SDF-2 production and spore formation in wild-type and mutant_Dictyostelium_ strains. Cells of the indicated wild-type (A) and mutant (B-E) strains were developed on filters (non-nutrient agar for _gpa4_-) and harvested at the mid-culmination stage. The fruiting bodies were dissociated and the cells washed before plating at 1×104 cells/cm2 in cAMP buffer. The cells were then treated with no addition (none), 10 pM synthetic SDF-2, 10 nM GABA, 10 units of SDF-3 or 100 nM hydrocortisone. Spores were counted after 1 hour. An aliquot of the supernatant was harvested for each sample to measure the amount of SDF-2 produced after purification using anion-exchange resin. Minus indicates production of less than 0.2 units of SDF-2/103 cells, whereas plus indicates more than 5000 units of SDF-2/103 cells.
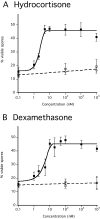
Fig. 4.
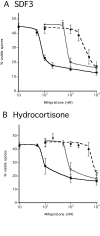
Sporulation induced by steroids. Developed KP cells were incubated with various concentrations of hydrocortisone or dexamethasone. The number of spores was determined after 1 hour of incubation. Each experiment was repeated three to five times. Antibodies against GABA (circle) or AcbA (open square) were added prior to the addition of (A) dexamethasone (black square) or (B) hydrocortisone (black square).
Fig. 5.
Competition between mifepristone and either hydrocortisone or SDF-3. KP cells were plated at low-density in cAMP buffer and incubated overnight. The indicated amounts of mifepristone and either SDF-3 (A) or hydrocortisone (B) were added. The number of spores was scored 1 hour later. One unit (black square), 10 units (open square), or 100 units (circle) of SDF-3 were added together with the indicated amounts of mifepristone. Hydrocortisone was added to 10 nM (black square), 100 nM (open square) or 1 μM (circle) together with the indicated amounts of mifepristone. Each experiment was repeated at least three times.
Fig. 6.
SDF-3 and hydrocortisone decrease the level of GABA required to overcome glutamate inhibition. Low-density KP cells were incubated overnight in cAMP buffer before addition of 1 mM glutamate. The indicated amount of GABA was then added with 2 units of SDF-3 (black circle) or 10 nM hydrocortisone (open circle) or left untreated (black square). Spores were counted after 1 hour. Each experiment was repeated at least three times. Almost identical results were obtained in the presence of 10 mM glutamate.
Fig. 7.
Involvement of GABA transaminase in SDF-2 production. (A) Low-density KP cells were incubated overnight (∼18 hours) before the addition of the indicated compounds. The following concentrations were used: 1 μM vigabatrin, 10 units of SDF-3, 10 nM CGP55845, 1/5000 anti-GABA antibodies (final dilution), 1/500 anti-AcbA antibodies (final dilution). The number of spores was scored 1 hour after the addition of SDF-3 or 2 hours after the addition of vigabatrin. An aliquot of the cell supernatants was harvested and SDF-2 purified using anion-exchange resin before quantification on fresh KP cells. (B) Low-density _gabT_-null KP cells lacking functional GABA transaminase were incubated for 17 hours in cAMP buffer. The number of spores was scored before and again 3 hours after addition of 1/5000 anti-GABA antibodies (final dilution), 1/500 anti-AcbA antibodies (final dilution), or 10 nM CGP55845. Aliquots of the cell supernatants were harvested and SDF-2 purified using anion-exchange resin before quantification on fresh KP cells. Minus indicates production of less than 0.2 units of SDF-2/103 cells, whereas plus indicates more than 5000 units of SDF-2/103 cells.
Similar articles
- Genetic evidence that the acyl coenzyme A binding protein AcbA and the serine protease/ABC transporter TagA function together in Dictyostelium discoideum cell differentiation.
Cabral M, Anjard C, Loomis WF, Kuspa A. Cabral M, et al. Eukaryot Cell. 2006 Dec;5(12):2024-32. doi: 10.1128/EC.00287-05. Epub 2006 Oct 20. Eukaryot Cell. 2006. PMID: 17056744 Free PMC article. - GABA induces terminal differentiation of Dictyostelium through a GABAB receptor.
Anjard C, Loomis WF. Anjard C, et al. Development. 2006 Jun;133(11):2253-61. doi: 10.1242/dev.02399. Epub 2006 May 3. Development. 2006. PMID: 16672332 - Loss of cAMP-specific phosphodiesterase rescues spore development in G protein mutant in dictyostelium.
Schwebs DJ, Nguyen HN, Miller JA, Hadwiger JA. Schwebs DJ, et al. Cell Signal. 2014 Feb;26(2):409-18. doi: 10.1016/j.cellsig.2013.10.003. Cell Signal. 2014. PMID: 24511612 Free PMC article. - Cell signaling during development of Dictyostelium.
Loomis WF. Loomis WF. Dev Biol. 2014 Jul 1;391(1):1-16. doi: 10.1016/j.ydbio.2014.04.001. Epub 2014 Apr 12. Dev Biol. 2014. PMID: 24726820 Free PMC article. Review. - Proteomics opens doors to the mechanisms of developmentally regulated secretion.
Alexander S, Srinivasan S, Alexander H. Alexander S, et al. Mol Cell Proteomics. 2003 Nov;2(11):1156-63. doi: 10.1074/mcp.R300011-MCP200. Epub 2003 Sep 22. Mol Cell Proteomics. 2003. PMID: 14504294 Review.
Cited by
- Proteomic and Transcriptomic Profiling Identifies Early Developmentally Regulated Proteins in Dictyostelium Discoideum.
González-Velasco Ó, De Las Rivas J, Lacal J. González-Velasco Ó, et al. Cells. 2019 Oct 1;8(10):1187. doi: 10.3390/cells8101187. Cells. 2019. PMID: 31581556 Free PMC article. - Unconventional secretion of Acb1 is mediated by autophagosomes.
Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V. Duran JM, et al. J Cell Biol. 2010 Feb 22;188(4):527-36. doi: 10.1083/jcb.200911154. Epub 2010 Feb 15. J Cell Biol. 2010. PMID: 20156967 Free PMC article. - Extracellular signaling in Dictyostelium.
Consalvo KM, Rijal R, Tang Y, Kirolos SA, Smith MR, Gomer RH. Consalvo KM, et al. Int J Dev Biol. 2019;63(8-9-10):395-405. doi: 10.1387/ijdb.190259rg. Int J Dev Biol. 2019. PMID: 31840778 Free PMC article. Review. - Evolution of developmental cyclic adenosine monophosphate signaling in the Dictyostelia from an amoebozoan stress response.
Schaap P. Schaap P. Dev Growth Differ. 2011 May;53(4):452-62. doi: 10.1111/j.1440-169X.2011.01263.x. Dev Growth Differ. 2011. PMID: 21585352 Free PMC article. Review. - The Galpha4 G protein subunit interacts with the MAP kinase ERK2 using a D-motif that regulates developmental morphogenesis in Dictyostelium.
Nguyen HN, Hadwiger JA. Nguyen HN, et al. Dev Biol. 2009 Nov 15;335(2):385-95. doi: 10.1016/j.ydbio.2009.09.011. Epub 2009 Sep 15. Dev Biol. 2009. PMID: 19765570 Free PMC article.
References
- Anjard, C. and Loomis, W. F. (2006). GABA induces terminal differentiation of Dictyostelium through a GABAB type receptor. Development 113, 2253-2261. - PubMed
- Anjard, C. and Loomis, W. F. (2008). Cytokinins induce sporulation in Dictyostelium. Development 135, 819-827. - PubMed
- Anjard, C., Chang, W. T., Gross, J. and Nellen, W. (1998a). Production and activity of spore differentiation factors (SDFs) in Dictyostelium. Development 125, 4067-4075. - PubMed
- Anjard, C., Zeng, C., Loomis, W. F. and Nellen, W. (1998b). Signal transduction pathways leading to spore differentiation in Dictyostelium discoideum. Dev. Biol. 193, 146-155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases