Soluble thrombomodulin protects ischemic kidneys - PubMed (original) (raw)
Soluble thrombomodulin protects ischemic kidneys
Asif A Sharfuddin et al. J Am Soc Nephrol. 2009 Mar.
Abstract
Altered coagulation and inflammation contribute to the pathogenesis of ischemic renal injury. Thrombomodulin is a necessary factor in the anticoagulant protein C pathway and has inherent anti-inflammatory properties. We studied the effect of soluble thrombomodulin (sTM) in a hypoperfusion model of ischemic kidney injury. To markedly reduce infrarenal aortic blood flow and femoral arterial pressures, we clamped the suprarenal aorta of rats, occluding them 90%, for 60 min. Reversible acute kidney injury (AKI) occurred at 24 h in rats subjected to hypoperfusion. Histologic analysis at 24 h revealed acute tubular necrosis (ATN), and intravital two-photon microscopy showed flow abnormalities in the microvasculature and defects of endothelial permeability. Pretreatment with rat sTM markedly reduced both I-R-induced renal dysfunction and tubular histologic injury scores. sTM also significantly improved microvascular erythrocyte flow rates, reduced microvascular endothelial leukocyte rolling and attachment, and minimized endothelial permeability to infused fluorescence dextrans, assessed by intravital quantitative multiphoton microscopy. Furthermore, sTM administered 2 h after reperfusion protected against ischemia-induced renal dysfunction at 24 h and improved survival. By using an sTM variant, we also determined that the protective effects of sTM were independent of its ability to generate activated protein C. These data suggest that sTM may have therapeutic potential for ischemic AKI.
Figures
Figure 1.
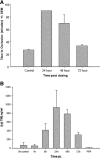
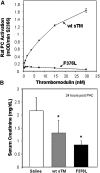
(A) Antithrombotic efficacy of 5-mg/kg SC dose of sTM in rat FeCl3 model. Time to occlusion was only monitored to 90 min. The 24-h time point never clotted. The 72-h mean time to occlusion was not significantly different from the control. Efficacy was declining after 48 h compared with 24 h and by 72 h there was not enough rat sTM remaining to be efficacious. Data represent the mean ± SD. (B) Pharmacokinetic data of sTM. Blood levels of sTM measured in FeCl3 model using ELISA after a 5-mg/kg SC dose. Peak blood levels were seen at 24 h. (n = 4). Data represent the mean ± SD.
Figure 2.
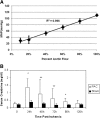
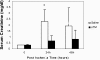
(A) MAP correlates with ABF. Linear regression analysis of three rats undergoing incremental increases in clamp intensity with measurement of real-time ABF and MAP. There was a high correlation of ABF with MAP, with an _R_2 = 0.996. (B) Rats undergoing PAC model ischemic-reperfusion injury have reduced kidney function when compared with sham-operated rats. Serum creatinine was significantly higher at 24 h (P < 0.005), 48 h (P < 0.05), 72 h (P < 0.05), and 96 h (P < 0.005) after PAC compared with serum creatinine in sham-operated rats (n = 10/group). **P < 0.01; *P < 0.05.
Figure 3.
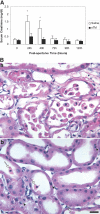
(A) sTM pretreatment ameliorates renal function impairment after PAC I-R injury. The increase in serum creatinine is abrogated by sTM (5 mg/kg) administered SC 24 h preischemia. Data represent mean ± SD. (*P < 0.005). n = 12/group from three separate experiments. (B) Histology in saline pretreated rat (a), and in sTM pretreated ischemic rats 24 h post-PAC. (b) Saline-pretreated rats exhibited marked loss of tubular cell into the lumen, interstitial edema, and reduced proximal tubule cell height as compared with sTM-treated rats. Note the relatively well preserved brush border in the sTM-treated rats. Arrows point to released PTCs, and the arrowhead points to amitotic cell. Bar = 20 μm.
Figure 4.
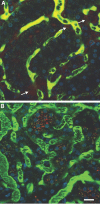
Microvascular blood flow in saline-pretreated and sTM-pretreated rats 24 h after PAC. Using real-time intravital two-photon microscopy, microvascular blood flow was assessed. Signal void in the vasculature represents the relative rapid movement of cellular structures (white blood cells, RBCs) in the vasculature streaming compared with the acquisition speed of the image. (A) Saline-pretreated rats 24 h post-PAC exhibited sluggish microvascular blood flow in most areas, with evidence of turbulence and rouleux formation (arrowhead) in any areas. Casts are also seen in the distal tubule (white arrow). (B) sTM-pretreated rats exhibited normal rapid microvascular blood flow in most areas 24 h post-PAC. Bar = 20 μm. (C) Mean blood flow velocity per vessel. sTM-treated rats had significantly improved blood flow velocities as measured by modified RBC line-scan method as compared with saline-treated animals (786.75 ± 280.75 μm/s versus 253.36 ± 95.01 μm/s P value < 0.05).
Figure 5.
Leukocyte adhesion to endothelium in a larger venule (arrows) in a saline-treated rat (see Supplemental Movie M3). Bar = 20 μm.
Figure 6.
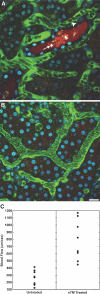
Endothelial permeability defect in saline-pretreated ischemic rat compared with sTM-pretreated ischemic rats. The large FITC-labeled dextran is generally retained in the vascular space and identifies the microvasculature, whereas the small rhodamine-labeled dextran is filtered at the glomerulus. Areas of extravasation of the small dextrans (arrows) from the vascular space into the interstitium were noted to be much more frequent and intense in (A) saline-pretreated rats as compared with (B) sTM-pretreated rats. Bar = 20 μm.
Figure 7.
sTM attenuates renal function impairment after PAC I-R injury when given 2 h postreperfusion at a dose of 1 mg/kg IV along with a simultaneous dose of 5 mg/kg SC. Significant renal protection was seen at 24 h (*P < 0.05; n = 6/group).
Figure 8.
Effect of sTM variant (F376L) on rat PC activation and efficacy in the PAC model. (A) Comparison of cofactor activity of human wt-sTM and variant F376L on thrombin-dependent activation of rat PC, measured by substrate S2366. Results are the mean ± SD (n = 3). (B) Effect of wt-sTM (5 mg/kg) and variant F376L (5 mg/kg) on serum creatinine at 24 h after PAC I-R injury in the rat (mean ± SD, *P < 0.05 versus saline control; n = 7).
Similar articles
- Activated protein C ameliorates LPS-induced acute kidney injury and downregulates renal INOS and angiotensin 2.
Gupta A, Rhodes GJ, Berg DT, Gerlitz B, Molitoris BA, Grinnell BW. Gupta A, et al. Am J Physiol Renal Physiol. 2007 Jul;293(1):F245-54. doi: 10.1152/ajprenal.00477.2006. Epub 2007 Apr 4. Am J Physiol Renal Physiol. 2007. PMID: 17409278 - Targeting thrombomodulin to circulating red blood cells augments its protective effects in models of endotoxemia and ischemia-reperfusion injury.
Carnemolla R, Villa CH, Greineder CF, Zaitsev S, Patel KR, Kowalska MA, Atochin DN, Cines DB, Siegel DL, Esmon CT, Muzykantov VR. Carnemolla R, et al. FASEB J. 2017 Feb;31(2):761-770. doi: 10.1096/fj.201600912R. Epub 2016 Nov 11. FASEB J. 2017. PMID: 27836986 Free PMC article. - Levels of protein C and soluble thrombomodulin in critically ill patients with acute kidney injury: a multicenter prospective observational study.
Bouchard J, Malhotra R, Shah S, Kao YT, Vaida F, Gupta A, Berg DT, Grinnell BW, Stofan B, Tolwani AJ, Mehta RL. Bouchard J, et al. PLoS One. 2015 Mar 19;10(3):e0120770. doi: 10.1371/journal.pone.0120770. eCollection 2015. PLoS One. 2015. PMID: 25790110 Free PMC article. - Soluble thrombomodulin, plasma-derived unactivated protein C, and recombinant human activated protein C in sepsis.
Dhainaut JF, Yan SB, Cariou A, Mira JP. Dhainaut JF, et al. Crit Care Med. 2002 May;30(5 Suppl):S318-24. doi: 10.1097/00003246-200205001-00023. Crit Care Med. 2002. PMID: 12004254 Review. - [Thrombomodulin].
Ohmori Y, Takahashi Y. Ohmori Y, et al. Nihon Yakurigaku Zasshi. 2000 Nov;116(5):283-9. Nihon Yakurigaku Zasshi. 2000. PMID: 11215378 Review. Japanese.
Cited by
- Two-photon imaging within the murine thorax without respiratory and cardiac motion artifact.
Presson RG Jr, Brown MB, Fisher AJ, Sandoval RM, Dunn KW, Lorenz KS, Delp EJ, Salama P, Molitoris BA, Petrache I. Presson RG Jr, et al. Am J Pathol. 2011 Jul;179(1):75-82. doi: 10.1016/j.ajpath.2011.03.048. Epub 2011 May 7. Am J Pathol. 2011. PMID: 21703395 Free PMC article. - Transcription Factor Trps1 Promotes Tubular Cell Proliferation after Ischemia-Reperfusion Injury through cAMP-Specific 3',5'-Cyclic Phosphodiesterase 4D and AKT.
Ju-Rong Y, Ke-Hong C, Kun H, Bi-Qiong F, Li-Rong L, Jian-Guo Z, Kai-Long L, Ya-Ni H. Ju-Rong Y, et al. J Am Soc Nephrol. 2017 Feb;28(2):532-544. doi: 10.1681/ASN.2016010009. Epub 2016 Jul 27. J Am Soc Nephrol. 2017. PMID: 27466160 Free PMC article. - Emerging therapeutic targets of sepsis-associated acute kidney injury.
Swaminathan S, Rosner MH, Okusa MD. Swaminathan S, et al. Semin Nephrol. 2015 Jan;35(1):38-54. doi: 10.1016/j.semnephrol.2015.01.005. Semin Nephrol. 2015. PMID: 25795498 Free PMC article. Review. - Magnetic resonance imaging with hyperpolarized [1,4-(13)C2]fumarate allows detection of early renal acute tubular necrosis.
Clatworthy MR, Kettunen MI, Hu DE, Mathews RJ, Witney TH, Kennedy BW, Bohndiek SE, Gallagher FA, Jarvis LB, Smith KG, Brindle KM. Clatworthy MR, et al. Proc Natl Acad Sci U S A. 2012 Aug 14;109(33):13374-9. doi: 10.1073/pnas.1205539109. Epub 2012 Jul 26. Proc Natl Acad Sci U S A. 2012. PMID: 22837393 Free PMC article. - Pathophysiology of acute kidney injury.
Basile DP, Anderson MD, Sutton TA. Basile DP, et al. Compr Physiol. 2012 Apr;2(2):1303-53. doi: 10.1002/cphy.c110041. Compr Physiol. 2012. PMID: 23798302 Free PMC article. Review.
References
- Bonventre JV, Weinberg JM: Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003 - PubMed
- Molitoris BA: Transitioning to therapy in ischemic acute renal failure. J Am Soc Nephrol 14: 265–267, 2003 - PubMed
- Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, Allison JJ: Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med 168: 609–616, 2008 - PubMed
- Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16: 162–168, 2005 - PubMed
- Mason J, Joeris B, Welsch J, Kriz W: Vascular congestion in ischemic renal failure: The role of cell swelling. Miner Electrolyte Metab 15: 114–124, 1989 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources