A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides - PubMed (original) (raw)
. 2009 Jan 28;29(4):918-29.
doi: 10.1523/JNEUROSCI.3952-08.2009.
Yao Huang, Fenqin Xue, Alain Simard, Jamie DeChon, Guohui Li, Jianliang Zhang, Linda Lucero, Min Wang, Michael Sierks, Gang Hu, Yongchang Chang, Ronald J Lukas, Jie Wu
Affiliations
- PMID: 19176801
- PMCID: PMC2857410
- DOI: 10.1523/JNEUROSCI.3952-08.2009
A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides
Qiang Liu et al. J Neurosci. 2009.
Abstract
Nicotinic acetylcholine receptors (nAChRs) containing alpha7 subunits are thought to assemble as homomers. alpha7-nAChR function has been implicated in learning and memory, and alterations of alpha7-nAChR have been found in patients with Alzheimer's disease (AD). Here we report findings consistent with a novel, naturally occurring nAChR subtype in rodent, basal forebrain cholinergic neurons. In these cells, alpha7 subunits are coexpressed, colocalize, and coassemble with beta2 subunit(s). Compared with homomeric alpha7-nAChRs from ventral tegmental area neurons, functional, presumably heteromeric alpha7beta2-nAChRs on cholinergic neurons freshly dissociated from medial septum/diagonal band (MS/DB) exhibit relatively slow kinetics of whole-cell current responses to nicotinic agonists and are more sensitive to the beta2 subunit-containing nAChR-selective antagonist, dihydro-beta-erythroidine (DHbetaE). Interestingly, presumed, heteromeric alpha7beta2-nAChRs are highly sensitive to functional inhibition by pathologically relevant concentrations of oligomeric, but not monomeric or fibrillar, forms of amyloid beta(1-42) (Abeta(1-42)). Slow whole-cell current kinetics, sensitivity to DHbetaE, and specific antagonism by oligomeric Abeta(1-42) also are characteristics of heteromeric alpha7beta2-nAChRs, but not of homomeric alpha7-nAChRs, heterologously expressed in Xenopus oocytes. Moreover, choline-induced currents have faster kinetics and less sensitivity to Abeta when elicited from MS/DB neurons derived from nAChR beta2 subunit knock-out mice rather than from wild-type mice. The presence of novel, functional, heteromeric alpha7beta2-nAChRs on basal forebrain cholinergic neurons and their high sensitivity to blockade by low concentrations of oligomeric Abeta(1-42) suggests possible mechanisms for deficits in cholinergic signaling that could occur early in the etiopathogenesis of AD and might be targeted by disease therapies.
Figures
Figure 1.
Identification of cholinergic neurons dissociated from basal forebrain. A, Phase-contrast image of a rat MS/DB brain slice [region confirmed according to the work of Paxinos and Watson (1986)]. MS/DB neurons (phase-contrast images of dissociated neurons; B) exhibited spontaneous action potential firing (C), insensitivity to muscarine (C), and action potential adaptation induced by depolarizing pulses (D) and did not show “sag”-like responses to hyperpolarizing pulses (E), suggesting they were cholinergic. MP, Membrane potential. F, Dissociated neuron (phase contrast, Ph) labeled with lucifer yellow (LY) showed positive ChAT immunostaining following patch-clamp recording.
Figure 2.
Native nAChR-mediated whole-cell current responses. An identified MS/DB cholinergic neuron (no hyperpolarization-induced current, I h) exhibited α7-nAChR-like current responses to 1 m
m
ACh and 10 m
m
choline (sensitive to blockade by 1 n
m
methyllycaconitine; MLA) but not to 0.1 m
m
RJR-2403, an agonist selective for α4β2-nAChRs (A), whereas an identified VTA DAergic neuron (evident I h) showed both α7-nAChR-like (i.e., choline and MLA-sensitive components) and α4β2-nAChR-like (i.e., RJR-2403-sensitive component) current responses (summed as in the response to ACh) (B). C, Typical traces of 10 m
m
choline-induced currents in MS/DB and VTA DAergic neurons showing different kinetics for current activation/desensitization with a slower response characteristic of MS/DB neurons. D, Statistical comparisons of kinetics of 10 m
m
choline-induced currents in MS/DB cholinergic and VTA DAergic neurons. ***p < 0.001.
Figure 3.
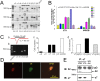
nAChR α7 and β2 subunits are coexpressed, colocalize, and coassemble in rat forebrain MS/DB neurons. RT-PCR products from whole brain and VTA and MS/DB regions (A) corresponding to the indicated nAChR subunits or to the housekeeping gene GAPDH were resolved on an agarose gel calibrated by the flanking 100 bp ladders (heavy band is 500 bp) and visualized using ethidium staining. Note that the representative gel shown for whole brain did not contain a sample for the nAChR α3 subunit RT-PCR product, which typically is similar in intensity to the sample on the gel for the VTA and MS/DB. B, Quantification of nAChR subunit mRNA levels for RT-PCR amplification followed by Southern hybridization with 32P-labeled, nested oligonucleotides normalized to the GAPDH internal control and to levels of each specific mRNA in whole rat brain (ordinate: ±SEM) for the indicated subunits. C, From 15 MS/DB neurons tested, after patch-clamp recordings (Ca: representative whole-cell current trace), the cell content was harvested and single-cell RT-PCR was performed, and the results show that α7 and β2 were the two major nAChR subunits naturally expressed in MS/DB cholinergic neurons (Cb–Cd). Double immunofluorescence labeling of a MS/DB neuron with anti-α7 and anti-β2 subunit antibodies revealed that α7 and β2 subunit proteins colocalized, and similar results were obtained using 31 neurons from 12 rats (D). Protein extracts from rat MS/DB (lane 1) or rat VTA (lane 2) or from MS/DB from nAChR β2 subunit knock-out (lane 4) or wild-type mice (lane 5) were immunoprecipitated (IP) with a rabbit anti-α7 antibody (Santa Cruz H302; lanes 1, 2, 4, and 5) or rabbit IgG as a control (lane 3). The eluted proteins from the precipitates were analyzed by immunoblotting (IB) with rat monoclonal anti-β2 subunit antibody mAb270 (top) or rabbit anti-α7 antiserum H302 (bottom). The β2 and α7 bands are indicated by arrows (E). All these data suggested that nAChR α7 and β2 nAChR subunits are coassembled in MS/DB neurons.
Figure 4.
Antagonist profiles for MS/DB and VTA nAChRs. Concentration-dependent block by MLA (at the indicated concentrations in n
m
after preexposure for 2 min and continued exposure during agonist application indicated by open bars) of 10 m
m
choline-induced (applied as indicated by closed bars) whole-cell currents (representative traces shown) in MS/DB (Aa) and VTA (Ab) neurons was not significantly different (p > 0.05, Ac). However, choline-induced currents in MS/DB neurons (Ba) were more sensitive to block by DHβE (at the indicated concentrations in μ
m
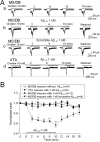
after preexposure for 2 min and continued exposure during agonist application indicated by open bars) than in VTA neurons (Bb; concentration–response profile shown in Bc). VH, Holding potential.
Figure 5.
Effects of 1 n
m
Aβ1–42 on α7β2-nAChRs on MS/DB neurons. Typical whole-cell current traces for responses of MS/DB neurons to 10 m
m
choline challenge at the indicated times after initial challenge alone show no detectable rundown during repetitive application of agonist (2 s exposure at 2 min intervals; Aa). Choline-induced currents in rat MS/DB neurons were suppressed by 1 n
m
Aβ1–42 (continuously applied for 10 min, but responses to challenges with choline are shown at the indicated times of Aβ exposure; Ab) but not by 1 n
m
scrambled Aβ1–42 (as a control; Ac). Choline-induced currents in VTA neurons were not affected by 1 n
m
Aβ1–42 (Ad). B, Normalized, mean (±SE), peak current responses (ordinate) as a function of time (abscissa, min) during challenges with choline alone (□), in the presence of 1 n
m
Aβ (▲), or in the presence of control, scrambled Aβ (▼) for the indicated numbers of MS/DB neurons, or during challenges with choline in the presence of 1 n
m
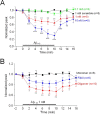
Aβ for the indicated number of VTA neurons (●) illustrate that only choline-induced currents in rat MS/DB neurons were sensitive to functional inhibition by Aβ. *p < 0.05; **p < 0.01.
Figure 6.
Inhibition of choline-induced currents in dissociated MS/DB neurons by Aβ1–42 was concentration and form dependent. A, Normalized mean (±SE) peak current responses (ordinate) of the indicated numbers of MS/DN neurons as a function of time (abscissa, min) during challenges with choline in the presence of 1 n
m
scrambled Aβ (■) or in the presence of 0.1 n
m
(●), 1 n
m
(▲), or 10 n
m
(▼) Aβ show concentration dependence of functional block. B, Normalized responses (ordinate) during challenges with choline in the presence of 1 n
m
monomeric (■), oligomeric (▲), or fibrillar (●) Aβ indicate insensitivity to monomeric Aβ and highest sensitivity to peptide oligomers. *p < 0.05, **p < 0.01, and ***p < 0.001.
Figure 7.
Effects of Aβ on heterologously expressed homomeric α7- and heteromeric α7β2-nAChRs in Xenopus oocytes. Choline (10 m
m
, 2 s exposure at 2 min intervals)-induced whole-cell current responses in oocytes injected with rat α7-nAChR subunit cRNA alone (Aa, black trace) or with α7 and β2 subunit cRNAs at a ratio of 1:1 (Aa, gray trace) show slower decay of elicited currents and a longer decay time constant for heteromeric receptors (Aa, Ab). The calibration bars represent 1 s and 1 μA for the α7-nAChR response (black trace) and 1 s and 100 nA for the α7β2-nAChR response (gray trace), thus also showing that current amplitudes were lower for heteromeric than for homomeric receptors. VH, Holding potential. B, Normalized mean (±SE) peak current responses (ordinate) of the indicated numbers of oocytes heterologously expressing nAChR α7 and β2 subunits (■, ●) or only α7 subunits (▲) as a function of time (abscissa, min) during challenges with choline alone (■) or in the presence of 10 n
m
Aβ (●, ▲) show sensitivity to functional block by Aβ only for heteromeric receptors. *p < 0.05, **p < 0.01, and ***p < 0.001.
Figure 8.
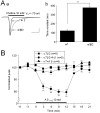
Kinetics, pharmacology, and Aβ sensitivity of α7-containing-nAChRs in nAChR β2 subunit knock-out mice. Genotype analyses demonstrated that nAChR β2 subunits are not expressed in nAChR β2 knock-out mice (A), whereas Lac-Z (as a marker for the knock-out) was absent in wild-type (WT) mice (B). Kinetic analyses showed that whole-cell current kinetics and amplitudes differed for MS/DB neurons from WT compared with nAChR β2 subunit knock-out homozygote mice (Ca, Cb). VH, Holding potential. Compared with MS/DB neurons from WT mice (Da), choline-induced currents in MS/DB (Db) neurons from β2 knock-outs were insensitive to DHβE but retained sensitivity to MLA (Dc). Aβ1–42 (1 n
m)
suppressed choline-induced currents in MS/DB neurons from WT (■) but not from β2 knock-out (●) mice (E). “Control” responses (▲) were choline-induced currents in neurons from WT mice without exposure to Aβ1–42. *p < 0.05, **p < 0.01.
Similar articles
- Functional α7β2 nicotinic acetylcholine receptors expressed in hippocampal interneurons exhibit high sensitivity to pathological level of amyloid β peptides.
Liu Q, Huang Y, Shen J, Steffensen S, Wu J. Liu Q, et al. BMC Neurosci. 2012 Dec 29;13:155. doi: 10.1186/1471-2202-13-155. BMC Neurosci. 2012. PMID: 23272676 Free PMC article. - The novel α7β2-nicotinic acetylcholine receptor subtype is expressed in mouse and human basal forebrain: biochemical and pharmacological characterization.
Moretti M, Zoli M, George AA, Lukas RJ, Pistillo F, Maskos U, Whiteaker P, Gotti C. Moretti M, et al. Mol Pharmacol. 2014 Sep;86(3):306-17. doi: 10.1124/mol.114.093377. Epub 2014 Jul 7. Mol Pharmacol. 2014. PMID: 25002271 Free PMC article. - A novel nicotinic mechanism underlies β-amyloid-induced neurotoxicity.
Liu Q, Xie X, Emadi S, Sierks MR, Wu J. Liu Q, et al. Neuropharmacology. 2015 Oct;97:457-63. doi: 10.1016/j.neuropharm.2015.04.025. Epub 2015 May 7. Neuropharmacology. 2015. PMID: 25959067 - Heteromeric α7β2 Nicotinic Acetylcholine Receptors in the Brain.
Wu J, Liu Q, Tang P, Mikkelsen JD, Shen J, Whiteaker P, Yakel JL. Wu J, et al. Trends Pharmacol Sci. 2016 Jul;37(7):562-574. doi: 10.1016/j.tips.2016.03.005. Epub 2016 May 11. Trends Pharmacol Sci. 2016. PMID: 27179601 Free PMC article. Review. - α7 nicotinic acetylcholine receptors in Alzheimer's disease: neuroprotective, neurotrophic or both?
Hernandez CM, Dineley KT. Hernandez CM, et al. Curr Drug Targets. 2012 May;13(5):613-22. doi: 10.2174/138945012800398973. Curr Drug Targets. 2012. PMID: 22300028 Review.
Cited by
- Nicotinic ACh receptors as therapeutic targets in CNS disorders.
Dineley KT, Pandya AA, Yakel JL. Dineley KT, et al. Trends Pharmacol Sci. 2015 Feb;36(2):96-108. doi: 10.1016/j.tips.2014.12.002. Epub 2015 Jan 29. Trends Pharmacol Sci. 2015. PMID: 25639674 Free PMC article. Review. - Mitochondria express α7 nicotinic acetylcholine receptors to regulate Ca2+ accumulation and cytochrome c release: study on isolated mitochondria.
Gergalova G, Lykhmus O, Kalashnyk O, Koval L, Chernyshov V, Kryukova E, Tsetlin V, Komisarenko S, Skok M. Gergalova G, et al. PLoS One. 2012;7(2):e31361. doi: 10.1371/journal.pone.0031361. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359587 Free PMC article. - Cellular response to β-amyloid neurotoxicity in Alzheimer's disease and implications in new therapeutics.
Zhang H, Li X, Wang X, Xu J, Elefant F, Wang J. Zhang H, et al. Animal Model Exp Med. 2023 Feb;6(1):3-9. doi: 10.1002/ame2.12313. Animal Model Exp Med. 2023. PMID: 36872303 Free PMC article. Review. - β-amyloid oligomers and prion protein: Fatal attraction?
Forloni G, Balducci C. Forloni G, et al. Prion. 2011 Jan-Mar;5(1):10-5. doi: 10.4161/pri.5.1.14367. Epub 2011 Jan 1. Prion. 2011. PMID: 21150333 Free PMC article. - Unbalanced Regulation of α7 nAChRs by Ly6h and NACHO Contributes to Neurotoxicity in Alzheimer's Disease.
Wu M, Liu CZ, Barrall EA, Rissman RA, Joiner WJ. Wu M, et al. J Neurosci. 2021 Oct 13;41(41):8461-8474. doi: 10.1523/JNEUROSCI.0494-21.2021. Epub 2021 Aug 26. J Neurosci. 2021. PMID: 34446574 Free PMC article.
References
- Azam L, Winzer-Serhan U, Leslie FM. Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience. 2003;119:965–977. - PubMed
- Burghaus L, Schütz U, Krempel U, de Vos RA, Jansen Steur EN, Wevers A, Lindstrom J, Schröder H. Quantitative assessment of nicotinic acetylcholine receptor proteins in the cerebral cortex of Alzheimer patients. Brain Res Mol Brain Res. 2000;76:385–388. - PubMed
- Counts SE, He B, Che S, Ikonomovic MD, DeKosky ST, Ginsberg SD, Mufson EJ. Alpha7 nicotinic receptor up-regulation in cholinergic basal forebrain neurons in Alzheimer disease. Arch Neurol. 2007;64:1771–1776. - PubMed
- Dineley KT, Xia X, Bui D, Sweatt JD, Zheng H. Accelerated plaque accumulation, associative learning deficits, and up-regulation of alpha 7 nicotinic receptor protein in transgenic mice co-expressing mutant human presenilin 1 and amyloid precursor proteins. J Biol Chem. 2002a;277:22768–22780. - PubMed
- Dineley KT, Bell KA, Bui D, Sweatt JD. beta-Amyloid peptide activates alpha 7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Biol Chem. 2002b;277:25056–25061. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous