The Parkinson disease protein leucine-rich repeat kinase 2 transduces death signals via Fas-associated protein with death domain and caspase-8 in a cellular model of neurodegeneration - PubMed (original) (raw)
The Parkinson disease protein leucine-rich repeat kinase 2 transduces death signals via Fas-associated protein with death domain and caspase-8 in a cellular model of neurodegeneration
Cherry Cheng-Ying Ho et al. J Neurosci. 2009.
Abstract
Neurodegenerative illnesses such as Parkinson and Alzheimer disease are an increasingly prevalent problem in aging societies, yet no therapies exist that retard or prevent neurodegeneration. Dominant missense mutations in leucine-rich repeat kinase 2 (LRRK2) are the most common genetic cause of Parkinson disease (PD), but the mechanisms by which mutant forms of LRRK2 disrupt neuronal function and cause cell death remain poorly understood. We report that LRRK2 interacts with the death adaptor Fas-associated protein with death domain (FADD), and that in primary neuronal culture LRRK2-mediated neurodegeneration is prevented by the functional inhibition of FADD or depletion of caspase-8, two key elements of the extrinsic cell death pathway. This pathway is activated by disease-triggering mutations, which enhance the LRRK2-FADD association and the consequent recruitment and activation of caspase-8. These results establish a direct molecular link between a mutant PD gene and the activation of programmed cell death signaling, and suggest that FADD/caspase-8 signaling contributes to LRRK2-induced neuronal death.
Figures
Figure 1.
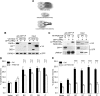
Parkinson disease mutations enhance the interaction between LRRK2 and FADD. A, Domain structure and Parkinson disease mutations of LRRK2. LRR, leucine-rich repeat; Roc, Ras of complex GTPase; COR, C-terminal of Roc. Five dominantly inherited PD-causing missense mutations are indicated. B, LRRK2 interacts with death adaptor proteins of the extrinsic pathway. 293T cells coexpressing GFP-LRRK2 and V5-tagged death adaptor proteins were subjected to anti-GFP immunoprecipitation followed by anti-GFP and anti-V5 immunoblotting. C, LRRK2 does not associate with death receptors. 293T cells coexpressing GFP-LRRK2 with GST-tagged cytoplasmic domains of death receptors were subjected to GST pull-down followed by immunoblotting. D, Enhanced association of FADD with LRRK2 PD mutants. 293T cells were cotransfected with wild-type (WT) or PD mutant GFP-LRRK2 and V5-tagged FADD. Anti-GFP immunoprecipitates were analyzed by anti-V5 and anti-GFP immunoblots. Ratios indicate the binding of FADD to LRRK2 relative to WT-LRRK2. E, PD mutations fail to enhance the association of LRRK2 with RIP1. 293T cells were cotransfected with V5-tagged RIP1 and WT or PD mutant GFP-LRRK2 and were assessed as in D. F, PD mutations fail to enhance the association of LRRK2 with TRADD. 293T cells were cotransfected with V5-tagged TRADD and WT or PD mutant GFP-LRRK2 and were assessed as in D. G, Endogenous LRRK2-FADD complex formation in mouse brain. Whole-brain lysates from 1-year-old wild-type mice were subjected to immunoprecipitation with anti-FADD (clone 7A2). Copurified LRRK2 was determined with anti-LRRK2 immunoblotting. H, Blocking LRRK2 kinase function prevents the enhanced FADD association with LRRK2 disease mutants. 293T cells expressing V5-tagged FADD and GFP-tagged WT or kinase-dead (KD) LRRK2 were immunoprecipitated and immunoblotted as in D.
Figure 2.
LRRK2-induced neuronal death requires FADD. A, A schematic depicts the domain structure of FADD, the isolated death domain (FADD-DD), and the leucine-zipper-DD (LZ-FADD-DD) in which the death domain is dimerized through the addition of a leucine zipper. B, FADD interacts with LRRK2 via its DD. GFP-LRRK2 was coexpressed with V5-tagged full-length FADD, FADD-DED, or FADD-DD in 293T cells, and was immunoprecipitated with anti-GFP. Copurified FADD or FADD domains were detected by anti-V5 immunoblotting. C, LRRK2-FADD interaction is enhanced by dimerization of FADD-DD. The interaction between GFP-LRRK2 and monomeric (DD) or dimeric (LZ) FADD-DD was assessed by anti-HA after immunoprecipitation with anti-GFP in 293T cells. D, FADD-DD is a poor inhibitor of LRRK2 neurotoxicity. Mouse cortical neurons were transfected with LRRK2 + lacZ (Ctrl) or LRRK2 + FADD-DD. A GFP reporter was cotransfected in each case. Transfected neurons displaying apoptotic nuclear morphology were counted 48 h after transfection using DAPI. Data are the mean ±SEM from three individual experiments of triplicate coverslips (n.s., nonsignificant; ANOVA with Tukey's post hoc test). E, Dimeric FADD-DD effectively blocks LRRK2 neurotoxicity. Mouse cortical neurons expressing LRRK + lacZ (Ctrl) or LRRK2 + LZ-FADD-DD were assessed as in D (***p < 0.001).
Figure 3.
LRRK2-induced neuronal death is caspase-8 (Casp8) dependent. A, FADD recruits caspase-8 to LRRK2. 293T cells were transfected with GFP-LRRK2, V5-FADD, and caspase-8 inactive mutant (C360S), as indicated. GFP-LRRK2 was immunoprecipitated, and copurified FADD and caspase-8 were detected by V5 and caspase-8 antibodies, respectively. B, Knockdown of caspase-8 blocks LRRK2-induced neurotoxicity. Mouse cortical neurons were incubated with Penetratin1-linked scrambled control (Ctrl) or caspase-8 siRNA 24 h before transfection with GFP-tagged wild-type (WT) or mutant LRRK2. Data are the mean ±SEM from three individual experiments of triplicate coverslips (*p < 0.05; **p < 0.01; ***p < 0.001). A representative immunoblot of caspase-8 levels after treatment with Penetratin1-linked siRNA is shown (inset). C, Knockdown of caspase-9 (Casp9) fails to prevent LRRK2-induced neuronal death. Caspase-9 RNAi, transfection, and neuronal death were performed and assessed as in B. D, Caspase-8 is selectively activated in brain tissue from patients with LRRK2 PD. Striatal lysates were analyzed by caspase-8 (clone 1C12), caspase-9, and caspase-1 (clone A19) immunoblotting. Caspase-2 was undetectable using three commercial antibodies (data not shown). The locations of the pro-caspase isoforms and their corresponding cleavage products are indicated (*nonspecific immunoreactive bands; #potential cleavage products with higher than expected mass: 37 and 35 kDa).
Similar articles
- A motif within the armadillo repeat of Parkinson's-linked LRRK2 interacts with FADD to hijack the extrinsic death pathway.
Antoniou N, Vlachakis D, Memou A, Leandrou E, Valkimadi PE, Melachroinou K, Re DB, Przedborski S, Dauer WT, Stefanis L, Rideout HJ. Antoniou N, et al. Sci Rep. 2018 Feb 22;8(1):3455. doi: 10.1038/s41598-018-21931-8. Sci Rep. 2018. PMID: 29472595 Free PMC article. - Mitogen-activated protein kinase kinase antagonized fas-associated death domain protein-mediated apoptosis by induced FLICE-inhibitory protein expression.
Yeh JH, Hsu SC, Han SH, Lai MZ. Yeh JH, et al. J Exp Med. 1998 Nov 16;188(10):1795-802. doi: 10.1084/jem.188.10.1795. J Exp Med. 1998. PMID: 9815257 Free PMC article. - (G2019S) LRRK2 activates MKK4-JNK pathway and causes degeneration of SN dopaminergic neurons in a transgenic mouse model of PD.
Chen CY, Weng YH, Chien KY, Lin KJ, Yeh TH, Cheng YP, Lu CS, Wang HL. Chen CY, et al. Cell Death Differ. 2012 Oct;19(10):1623-33. doi: 10.1038/cdd.2012.42. Epub 2012 Apr 27. Cell Death Differ. 2012. PMID: 22539006 Free PMC article. - LRRK2 and the "LRRKtosome" at the Crossroads of Programmed Cell Death: Clues from RIP Kinase Relatives.
Rideout HJ, Re DB. Rideout HJ, et al. Adv Neurobiol. 2017;14:193-208. doi: 10.1007/978-3-319-49969-7_10. Adv Neurobiol. 2017. PMID: 28353285 Review. - Contribution of GTPase activity to LRRK2-associated Parkinson disease.
Tsika E, Moore DJ. Tsika E, et al. Small GTPases. 2013 Jul-Sep;4(3):164-70. doi: 10.4161/sgtp.25130. Epub 2013 Jun 10. Small GTPases. 2013. PMID: 24025585 Free PMC article. Review.
Cited by
- Coordinate Regulation of Neurite Outgrowth by LRRK2 and Its Interactor, Rab5.
Heo HY, Kim KS, Seol W. Heo HY, et al. Exp Neurobiol. 2010 Sep;19(2):97-105. doi: 10.5607/en.2010.19.2.97. Epub 2010 Sep 30. Exp Neurobiol. 2010. PMID: 22110348 Free PMC article. - Biology of mitochondria in neurodegenerative diseases.
Martin LJ. Martin LJ. Prog Mol Biol Transl Sci. 2012;107:355-415. doi: 10.1016/B978-0-12-385883-2.00005-9. Prog Mol Biol Transl Sci. 2012. PMID: 22482456 Free PMC article. Review. - LRRK2; Communicative Role in the Treatment of Parkinson's Disease and Ulcerative Colitis Overlapping.
Lashgari NA, Roudsari NM, Niknejad A, Shamsnia HS, Shayan M, Shalmani LM, Momtaz S, Rezaei N, Abdolghaffari AH. Lashgari NA, et al. CNS Neurol Disord Drug Targets. 2024;23(10):1177-1188. doi: 10.2174/0118715273270874231205050727. CNS Neurol Disord Drug Targets. 2024. PMID: 38279762 Review. - Signal transduction protein array analysis links LRRK2 to Ste20 kinases and PKC zeta that modulate neuronal plasticity.
Zach S, Felk S, Gillardon F. Zach S, et al. PLoS One. 2010 Oct 7;5(10):e13191. doi: 10.1371/journal.pone.0013191. PLoS One. 2010. PMID: 20949042 Free PMC article. - Mitochondrial and Cell Death Mechanisms in Neurodegenerative Diseases.
Martin LJ. Martin LJ. Pharmaceuticals (Basel). 2010;3(4):839-915. doi: 10.3390/ph3040839. Pharmaceuticals (Basel). 2010. PMID: 21258649 Free PMC article.
References
- Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP, Green DR, Salvesen GS. A unified model for apical caspase activation. Mol Cell. 2003;11:529–541. - PubMed
- Bonifati V. LRRK2 low-penetrance mutations (Gly2019Ser) and risk alleles (Gly2385Arg)-linking familial and sporadic Parkinson disease. Neurochem Res. 2007;32:1700–1708. - PubMed
- Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5:521–531. - PubMed
- Chung CW, Hong YM, Song S, Woo HN, Choi YH, Rohn T, Jung YK. Atypical role of proximal caspase-8 in truncated Tau-induced neurite regression and neuronal cell death. Neurobiol Dis. 2003;14:557–566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous