Aberrant promoter methylation of SPARC in ovarian cancer - PubMed (original) (raw)
Aberrant promoter methylation of SPARC in ovarian cancer
Matthew J Socha et al. Neoplasia. 2009 Feb.
Abstract
Epigenetic silencing of tumor suppressor genes is a new focus of investigation in the generation and proliferation of carcinomas. Secreted protein acidic and rich in cysteine (SPARC) is reportedly detrimental to the growth of ovarian cancer cells and has been shown to be epigenetically silenced in several cancers. We hypothesized that SPARC is downregulated in ovarian cancer through aberrant promoter hypermethylation. To that end, we analyzed SPARC expression in ovarian cancer cell lines and investigated the methylation status of the Sparc promoter using methylation-specific polymerase chain reaction. Our results show that SPARC mRNA expression is decreased in three (33%) and absent in four (44%) of the nine ovarian cancer cell lines studied, which correlated with hypermethylation of the Sparc promoter. Treatment with the demethylating agent 5-aza-2'-deoxycytidine rescued SPARC mRNA and protein expression. Addition of exogenous SPARC, as well as ectopic expression by an adenoviral vector, resulted in decreased proliferation of ovarian cancer cell lines. Investigation of primary tumors revealed that the Sparc promoter is methylated in 68% of primary ovarian tumors and that the levels of SPARC protein decrease as the disease progresses from low to high grade. Lastly, de novo methylation of Sparc promoter was shown to be mediated by DNA methyltransferase 3a. These results implicate Sparc promoter methylation as an important factor in the genesis and survival of ovarian carcinomas and provide new insights into the potential use of SPARC as a novel biomarker and/or treatment modality for this disease.
Figures
Figure 1
SPARC expression is reduced in ovarian cancer cell lines caused by aberrant methylation. (A) Expression levels of SPARC measured by RT-PCR in nine ovarian cancer cell lines, one immortalized control cell line (HOSE 1–15), and three primary cell lines (NHOSE 56, 58, and 59). Pictures shown are representative of three independent experiments. (B) Expression of SPARC protein from nine ovarian cancer cell lines, two immortalized control cell lines (HOSE 1–15 and Meso 301), and three primary cell lines measured by Western blot. Blots shown are representative of three independent experiments. (C) Representative examples of MSP of the Sparc promoter in three ovarian cancer cell lines and two immortalized control cell lines. Results shown are representative of three independent experiments.
Figure 2
Restoration of Sparc mRNA and protein expression through global demethylation is independent of histone acetylation in vitro. (A) Expression of Sparc mRNA measured by RT-PCR from three representative ovarian cancer cell lines and two normal control cell lines before (right) and after (left) treatment with 5-Aza-CdR. Pictures shown are representative of three independent experiments. (B) Expression levels of SPARC protein measured by Western blot analysis in nine ovarian cancer cell lines and two normal control cell lines before (top) and after treatment (bottom) with 5-Aza-CdR. Blots shown are representative of three independent experiments. (C) Expression of SPARC mRNA as measured by RT-PCR from six representative ovarian cancer cell lines before (left) and after (right) treatment with TSA, a histone deacetylase inhibitor. These results confirm that, with the exception of DOV 13 cell line, histone acetylation seems to play no significant role in SPARC expression. Results shown are representative of three independent experiments.
Figure 3
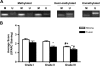
Sparc is aberrantly methylated in ovarian cancer tumor tissue, and this methylation results in an inverse correlation between disease stage and SPARC levels. (A) Methylation-specific PCR analysis of the Sparc promoter from laser-captured ovarian tumor samples. Images are representative of methylation, hemimethylation, and no methylation of the promoter. Pictures shown are representative of three independent experiments. (B) Graphical representation of the relative levels of SPARC protein present in tumor samples of varying grades. Images were blindly scored on a scale of 0 to 4 for SPARC staining intensity (*P < .05 vs stroma; †P < .05 vs grade I stroma; P < .05 vs grade II stroma; Ψ_p_ < .05 vs grade I tumor).
Figure 4
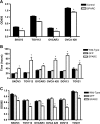
Exogenous SPARC inhibits proliferation of ovarian cancer in vitro. (A) BrdU incorporation in the absence or presence of exogenous SPARC (40 µg/ml) shows that in the four ovarian cancer cell lines, SPARC inhibits proliferation. Results shown are expressed as the mean ± SEM and were representative of two independent experiments performed in quadruplicates (*P < .05). (B) Measurements of the doubling time of six of the ovarian cancer cell lines showed significant increases after induction of SPARC production through an adenoviral vector (*P < .05). (C) MTS assay on the six ovarian cancer cell lines expressing SPARC after adenoviral infection shows a significant decrease in the proliferation rate of the cells. Results shown are expressed as the mean ± SEM and were representative of two independent experiments performed in triplicates (* P < .05).
Figure 5
DNMT3a associates with Sparc promoter in ovarian cancer cells. (A) Expression of DNMT3a mRNA measured by RT-PCR in nine ovarian cancer cell lines and one immortalized control cell line show that this DNMT isoform is upregulated in ovarian cancer cells. The human breast cancer cell line MCF7, overexpressing the DNMT3a isoform, was used as a positive control. Pictures shown are representative of three independent experiments. (B) Basal and 5-Aza-CdR-treated protein levels of SPARC and DNMT isoforms in SKOV3 and OVCAR3 cell lines. 5-Aza-CdR treatment resulted in a significant increase in SPARC expression, but only significantly reduced DNMT3a levels. (C) Protein band intensities from three independent experiments were quantified by densitometry. Changes in the band intensity of DNMT1, DNMT3a, and SPARC in 5-Aza-CdR-treated samples, relative to untreated controls (set at 100%), are expressed as the mean ± SEM. DNMT3a, but not DNMT1, was reduced significantly, whereas SPARC was increased significantly in 5-Aza-CdR-treated OVCAR3 (*P < .05) and SKOV3 cell lines (**P < .05) relative to controls. (D) Chromatin immunoprecipitation analysis in SKOV3 cells revealed a significant DNMT3a association with Sparc promoter, relative to IgG-negative controls (set as 1). RNA polymerase II (RNA Poly) association was used as a positive control for the assay. (E) Chromatin immunoprecipitation analysis in SKOV3 cells in the absence or presence of 5-Aza-CdR revealed a significant decrease only in DNMT3a levels relative to respective IgG-negative controls (set as 1). Results shown are representative of two independent experiments performed in triplicates (*P < .05 relative to IgG controls).
Similar articles
- SPARC promoter hypermethylation in colorectal cancers can be reversed by 5-Aza-2'deoxycytidine to increase SPARC expression and improve therapy response.
Cheetham S, Tang MJ, Mesak F, Kennecke H, Owen D, Tai IT. Cheetham S, et al. Br J Cancer. 2008 Jun 3;98(11):1810-9. doi: 10.1038/sj.bjc.6604377. Epub 2008 May 6. Br J Cancer. 2008. PMID: 18458674 Free PMC article. - Genome-wide methylation profiling of ovarian cancer patient-derived xenografts treated with the demethylating agent decitabine identifies novel epigenetically regulated genes and pathways.
Tomar T, de Jong S, Alkema NG, Hoekman RL, Meersma GJ, Klip HG, van der Zee AG, Wisman GB. Tomar T, et al. Genome Med. 2016 Oct 20;8(1):107. doi: 10.1186/s13073-016-0361-5. Genome Med. 2016. PMID: 27765068 Free PMC article. - Aberrant methylation of the SPARC gene promoter and its clinical implication in gastric cancer.
Chen ZY, Zhang JL, Yao HX, Wang PY, Zhu J, Wang W, Wang X, Wan YL, Chen SW, Chen GW, Liu YC. Chen ZY, et al. Sci Rep. 2014 Dec 17;4:7035. doi: 10.1038/srep07035. Sci Rep. 2014. PMID: 25516351 Free PMC article. - DNA demethylation and invasive cancer: implications for therapeutics.
Cheishvili D, Boureau L, Szyf M. Cheishvili D, et al. Br J Pharmacol. 2015 Jun;172(11):2705-15. doi: 10.1111/bph.12885. Epub 2015 Apr 27. Br J Pharmacol. 2015. PMID: 25134627 Free PMC article. Review. - Epigenetic DNA-(cytosine-5-carbon) modifications: 5-aza-2'-deoxycytidine and DNA-demethylation.
Patra SK, Bettuzzi S. Patra SK, et al. Biochemistry (Mosc). 2009 Jun;74(6):613-9. doi: 10.1134/s0006297909060042. Biochemistry (Mosc). 2009. PMID: 19645665 Review.
Cited by
- Roles of SPARC in urothelial carcinogenesis, progression and metastasis.
Said N. Said N. Oncotarget. 2016 Oct 11;7(41):67574-67585. doi: 10.18632/oncotarget.11590. Oncotarget. 2016. PMID: 27564266 Free PMC article. Review. - Associations of tumor suppressor SPARCL1 with cancer progression and prognosis.
Li T, Liu X, Yang A, Fu W, Yin F, Zeng X. Li T, et al. Oncol Lett. 2017 Sep;14(3):2603-2610. doi: 10.3892/ol.2017.6546. Epub 2017 Jul 8. Oncol Lett. 2017. PMID: 28927026 Free PMC article. - Dinosaurs and ancient civilizations: reflections on the treatment of cancer.
Rehemtulla A. Rehemtulla A. Neoplasia. 2010 Dec;12(12):957-68. doi: 10.1593/neo.101588. Neoplasia. 2010. PMID: 21170260 Free PMC article. - The novel, small-molecule DNA methylation inhibitor SGI-110 as an ovarian cancer chemosensitizer.
Fang F, Munck J, Tang J, Taverna P, Wang Y, Miller DF, Pilrose J, Choy G, Azab M, Pawelczak KS, VanderVere-Carozza P, Wagner M, Lyons J, Matei D, Turchi JJ, Nephew KP. Fang F, et al. Clin Cancer Res. 2014 Dec 15;20(24):6504-16. doi: 10.1158/1078-0432.CCR-14-1553. Epub 2014 Oct 14. Clin Cancer Res. 2014. PMID: 25316809 Free PMC article. - Clinical significance of epigenetic silencing and re-expression of O6-methylguanine-DNA methyltransferase using epigenetic agents in laryngeal carcinoma.
Yang J, Zhu XB, He LX, Gu ZW, Jin MZ, Ji WY. Yang J, et al. Oncol Lett. 2015 Jan;9(1):35-42. doi: 10.3892/ol.2014.2662. Epub 2014 Nov 3. Oncol Lett. 2015. PMID: 25452816 Free PMC article.
References
- Ibanez de Caceres I, Battagli C, Esteller M, Herman JG, Dulaimi E, Edelson MI, Bergman C, Ehya H, Eisenberg BL, Cairns P. Tumor cell-specific BRCA1 and RASSF1A hypermethylation in serum, plasma, and peritoneal fluid from ovarian cancer patients. Cancer Res. 2004;64:6476–6481. - PubMed
- Makarla PB, Saboorian MH, Ashfaq R, Toyooka KO, Toyooka S, Minna JD, Gazdar AF, Schorge JO. Promoter hypermethylation profile of ovarian epithelial neoplasms. Clin Cancer Res. 2005;11:5365–5369. - PubMed
- Teodoridis JM, Hall J, Marsh S, Kannall HD, Smyth C, Curto J, Siddiqui N, Gabra H, McLeod HL, Strathdee G, et al. CpG island methylation of DNA damage response genes in advanced ovarian cancer. Cancer Res. 2005;65:8961–8967. - PubMed
- Cai LY, Abe M, Izumi S, Imura M, Yasugi T, Ushijima T. Identification of PRTFDC1 silencing and aberrant promoter methylation of GPR150, ITGA8 and HOXD11 in ovarian cancers. Life Sci. 2007;80:1458–1465. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous