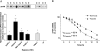Transcriptional regulation of urokinase-type plasminogen activator receptor by hypoxia-inducible factor 1 is crucial for invasion of pancreatic and liver cancer - PubMed (original) (raw)
Transcriptional regulation of urokinase-type plasminogen activator receptor by hypoxia-inducible factor 1 is crucial for invasion of pancreatic and liver cancer
Peter Büchler et al. Neoplasia. 2009 Feb.
Abstract
Angioinvasion is critical for metastasis with urokinase-type plasminogen activator receptor (uPAR) and tumor hypoxia-activated hypoxia-inducible factor 1 (HIF-1) as key players. Transcriptional control of uPAR expression by HIF has never been reported. The aim of the present study, therefore, was to test whether tumor hypoxia-induced HIF expression may be linked to transcriptional activation of uPAR and dependent angioinvasion. We used human pancreatic cancer cells and a model of parental and derived HIF-1beta-deficient mouse liver cancer cell lines and performed Northern blot analysis, nuclear runoff assays, electrophoretic mobility shift assay, polymerase chain reaction-generated deletion mutants, luciferase assays, Matrigel invasion assays, and in vivo angioinvasion assays in the chorioallantoic membrane of fertilized chicken eggs. Urokinase-type plasminogen activator receptor promoter analysis resulted in four putative HIF binding sites. Hypoxia strongly induced de novo transcription of uPAR mRNA. With sequential deletion mutants of the uPAR promoter, it was possible to identify one HIF binding site causing a nearly 200-fold increase in luciferase activity. Hypoxia enhanced the number of invading tumor cells in vitro and in vivo. In contrast, HIF-1beta-deficient cells failed to upregulate uPAR expression, to activate luciferase activity, and to invade on hypoxia. Taken together, we show for the first time that uPAR is under transcriptional control of HIF and that this is important for hypoxia-induced metastasis.
Figures
Figure 1
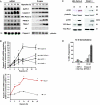
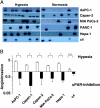
(A) Hypoxia enhances uPAR expression in Northern blot and Western blot experiments. Human pancreatic cancer cell lines AsPC-1, Capan-2, MIA PaCa-2, and PANC-1 together with the murine hepatoma cell line Hepa-1c1c7 and its mutated cell clone c4, lacking functional HIF-1β, were cultured under normoxic or hypoxic conditions. After 24 hours, total RNA and protein were isolated, size-fractionated, and transferred to membranes, which were hybridized in Northern blot experiments with a cDNA probe containing 500 bp of the human uPAR gene or in Western blot experiments with uPAR antibody 3932. The 7 S and γ-tubulin probes served as controls to demonstrate equal loading. (B) Densitometry of hypoxia-induced uPAR expression was done as described in the Materials and Methods section. The signal intensity of untreated normoxic control cells was measured and defined as a relative optical density of 1.0. On the basis of the individual signal intensity, the increase or the decrease of the relative optical density in comparison with untreated normoxic cells was determined. *P < .05. (C) Cell signaling factors known to be downstream of uPAR were analyzed. MIA PaCa-2 and PANC-1 cells were cultured for 16 hours in 21% O2 (N) or 1.0% O2 (H). Cell extracts were affinity-precipitated and subjected to immunoblot analysis to detect GTP-bound Rac1. The original cell extracts were studied by immunoblot analysis to determine total Rac1. Cell extracts were also probed for phosphorylated ERK/MAPK and uPAR. (D) DNA fragmentation assay. AsPC-1, Capan-2, MIA PaCa-2, and PANC-1 were incubated with 40 nM gemcitabine, and 72 hours later, apoptosis was determined by Nicoletti staining of fragmented DNA and FACS analysis.
Figure 2
Hypoxia enhances uPAR transcription. (A) Top panel: Nuclear runoff assays comparing normoxic (N) and hypoxic (H) uPAR transcription. Immediately after reaching 80% confluence, cells were exposed to normoxia or hypoxia for 16 hours, and nuclei were prepared. [α-32P]UTP was incorporated in total RNA by in vitro transcription assays as described in the Materials and Methods section. The radioactive-labeled RNA samples were hybridized to cDNA specific for uPAR, and autoradiography was performed. Lower panel: The signal intensity of normoxic control cells was defined as a relative optical density of 1.0. On the basis of the individual signal intensity, the increase or the decrease of the relative optical density in comparison with untreated normoxic cells was determined. *P < .05. (B) Effect of hypoxia on mRNA stability in MIA PaCa-2 cells. RNA was isolated from cells cultured under normoxic and hypoxic conditions after incubating with actinomycin D (5 mg/_µ_l) for the indicated time. Quantitative reverse transcription-polymerase chain reaction was performed and uPAR mRNA-quantified. Values of control cells were set at 100%. Each graph represents mean ± SEM for three independent experiments.
Figure 3
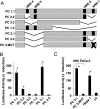
The consensus HRE within the uPAR promoter regulates hypoxia-induced promoter activity. (A) Scheme of the human uPAR promoter including putative consensus HREs. The black bars mark putative HREs relative to the ATG codon. A series of deletion mutants were constructed, and the length of the constructs relative to the transcription start site is indicated. (B) MIA PaCA-2 cells were transfected as described in the Materials and Methods section. Transfected cells were maintained at 21°C and 1% O2 for 16 hours. Luciferase reporter gene assays were performed, and luciferase activities were normalized by using a dual-luciferase reporter system, in which relative firefly luciferase activities were calculated thus normalizing transfection efficiency according to the renilla luciferase activities. Values represent means ± SD of n = 3 experiments performed in duplicate. Statistical differences are indicated by asterisks (*P < .05, Student's paired t test). (C) MIA PaCa-2, Hepa-1, and c4 cells were transfected with the constructs indicated and analyzed as described above.
Figure 4
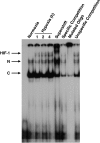
Hypoxia-inducible factor binds to the consensus HRE in uPAR promoter on hypoxia. MIA PaCa-2 cells were cultured under normoxia or hypoxia for 1, 2, and 4 hours. Nuclear proteins were harvested, and binding of a consensus HRE oligonucleotide of the uPAR promoter was analyzed by EMSA as described in the Materials and Methods section. Control experiments were performed with nuclear extracts from cells exposed 4 hours to normoxia or 1, 2, and 4 hours to hypoxia. For supershift and competition experiments, the extract from cells cultured for 4 hours under hypoxic conditions was used. The following reagents were added: anti-HIF-1α antibody (Supershift), a 30-fold excess of unlabeled consensus HRE oligonucleotide of the uPAR promoter (Specific Competition), a labeled mutant consensus HRE oligonucleotide of the uPAR promoter (Mutated Oligo), and a 30-fold excess of unlabeled mutant consensus HRE oligonucleotide of the uPAR promoter. C indicates constitutive; HIF-1, induced; N, nonspecific.
Figure 5
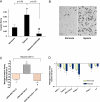
uPAR inhibition reduces tumor cell invasion. (A) MIA PaCa-2 cells were cultured under normoxic or hypoxic conditions as indicated. Invasion was measured by using a reconstituted basement membrane in Costar Transwell inserts containing a polycarbonate membrane with 8-mm pores in the presence of Matrigel as described in the Materials and Methods section. At 24 hours after incubation, cells invading through the semipermeable membrane in the presence or absence of blocking anti-uPAR antibody (uPAR-NAB) were fixed and stained. The invasion score was then determined by counting the total number of stained cells at the underside of the polycarbonate membranes under a microscope. Error bars, SEM across three experiments. (B) An example of the underside of a membrane showing invading cells by light microscopy is displayed for cells treated with normoxia or hypoxia as indicated. (C) Stably transfected MIA PaCa-2 cells expressing uPAR-siRNA or uPAR full-length cDNA were also cultured 24 hours under low oxygen levels. Clearly, the uPAR expression was associated with tumor cell invasion. Overexpression of uPAR could be reverted by the addition uPAR-NAB. (D) siRNA treatment reduced tumor cell invasion in all cell lines both under normoxic and hypoxic culture conditions. For control treatment, a scrambled siRNA oligonucleotide was used. Values represent means ± SD of n = 3 experiments performed in duplicate (*P < .05, Student's paired t test; #P < .05 compared with hypoxia [A] or uPAR overexpression [D]).
Figure 6
uPAR inhibition blocks angioinvasion after hypoxia in vivo. (A) Cells were inoculated onto the dropped CAMs of chicken embryos. Three days later, the lower CAMs were excised, genomic DNA was isolated, and 1 _µ_g of DNA was used to amplify human Alu sequences in the presence of [32P]-dCTP. Polymerase chain reaction products were analyzed by PAGE and visualized by autoradiography. (B) Inhibition of intravasation by a neutralizing uPAR antibody. Inoculated tumor cells were repeatedly flushed with an anoxic gas mixture for the induction of hypoxia. For uPAR inhibition, 40 mg/ml neutralizing uPAR antibody was added ontopically (Materials and Methods section). Alu sequences were analyzed as described above. All experiments were repeated at least three times with 10 eggs in each group. Angioinvasion was calculated relative to normal untreated cells, which were set as 1. *P < .05, compared hypoxia versus normoxia as well as hypoxia versus uPAR inhibition.
Similar articles
- Hypoxia inducible factor-1 mediates upregulation of urokinase-type plasminogen activator receptor gene transcription during hypoxia in cervical cancer cells.
Nishi H, Sasaki T, Nagamitsu Y, Terauchi F, Nagai T, Nagao T, Isaka K. Nishi H, et al. Oncol Rep. 2016 Feb;35(2):992-8. doi: 10.3892/or.2015.4449. Epub 2015 Nov 25. Oncol Rep. 2016. PMID: 26718775 - Hypoxia Promotes Extravillous Trophoblast Cell Invasion through the Hypoxia-Inducible Factor Urokinase-Type Plasminogen Activator Receptor Pathway.
Shigemitsu A, Naruse K, Kobayashi H. Shigemitsu A, et al. Gynecol Obstet Invest. 2022;87(3-4):232-241. doi: 10.1159/000525851. Epub 2022 Jul 4. Gynecol Obstet Invest. 2022. PMID: 35785760 - FOXM1c promotes pancreatic cancer epithelial-to-mesenchymal transition and metastasis via upregulation of expression of the urokinase plasminogen activator system.
Huang C, Xie D, Cui J, Li Q, Gao Y, Xie K. Huang C, et al. Clin Cancer Res. 2014 Mar 15;20(6):1477-88. doi: 10.1158/1078-0432.CCR-13-2311. Epub 2014 Jan 22. Clin Cancer Res. 2014. PMID: 24452790 Free PMC article. - The role and regulation of urokinase-type plasminogen activator receptor gene expression in cancer invasion and metastasis.
Wang Y. Wang Y. Med Res Rev. 2001 Mar;21(2):146-70. doi: 10.1002/1098-1128(200103)21:2<146::aid-med1004>3.0.co;2-b. Med Res Rev. 2001. PMID: 11223863 Review. - Carotenoids in Cancer Metastasis-Status Quo and Outlook.
Koklesova L, Liskova A, Samec M, Zhai K, Abotaleb M, Ashrafizadeh M, Brockmueller A, Shakibaei M, Biringer K, Bugos O, Najafi M, Golubnitschaja O, Büsselberg D, Kubatka P. Koklesova L, et al. Biomolecules. 2020 Dec 10;10(12):1653. doi: 10.3390/biom10121653. Biomolecules. 2020. PMID: 33321708 Free PMC article. Review.
Cited by
- Dinosaurs and ancient civilizations: reflections on the treatment of cancer.
Rehemtulla A. Rehemtulla A. Neoplasia. 2010 Dec;12(12):957-68. doi: 10.1593/neo.101588. Neoplasia. 2010. PMID: 21170260 Free PMC article. - Regulation of G(1) arrest and apoptosis in hypoxia by PERK and GCN2-mediated eIF2alpha phosphorylation.
Liu Y, László C, Liu Y, Liu W, Chen X, Evans SC, Wu S. Liu Y, et al. Neoplasia. 2010 Jan;12(1):61-8. doi: 10.1593/neo.91354. Neoplasia. 2010. PMID: 20072654 Free PMC article. - Epigenetic Regulation During Hypoxia and Its Implications in Cancer.
Pant D, Mutnuru SA, Shukla S. Pant D, et al. Subcell Biochem. 2022;100:361-390. doi: 10.1007/978-3-031-07634-3_11. Subcell Biochem. 2022. PMID: 36301500 - The War on Cancer rages on.
Rehemtulla A. Rehemtulla A. Neoplasia. 2009 Dec;11(12):1252-63. doi: 10.1593/neo.91866. Neoplasia. 2009. PMID: 20019833 Free PMC article. - Modulation of Cellular Function by the Urokinase Receptor Signalling: A Mechanistic View.
Alfano D, Franco P, Stoppelli MP. Alfano D, et al. Front Cell Dev Biol. 2022 Apr 8;10:818616. doi: 10.3389/fcell.2022.818616. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35493073 Free PMC article. Review.
References
- Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. - PubMed
- Hoffmann AC, Mori R, Vallbohmer D, Brabender J, Klein E, Drebber U, Baldus SE, Cooc J, Azuma M, Metzger R, et al. High expression of HIF1a is a predictor of clinical outcome in patients with pancreatic ductal adenocarcinomas and correlated to PDGFA, VEGF, and bFGF. Neoplasia. 2008;10:674–679. - PMC - PubMed
- Buchler P, Reber HA, Buchler M, Shrinkante S, Buchler MW, Friess H, Semenza GL, Hines OJ. Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas. 2003;26:56–64. - PubMed
- Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and PO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical