Stretching single talin rod molecules activates vinculin binding - PubMed (original) (raw)
Stretching single talin rod molecules activates vinculin binding
Armando del Rio et al. Science. 2009.
Abstract
The molecular mechanism by which a mechanical stimulus is translated into a chemical response in biological systems is still unclear. We show that mechanical stretching of single cytoplasmic proteins can activate binding of other molecules. We used magnetic tweezers, total internal reflection fluorescence, and atomic force microscopy to investigate the effect of force on the interaction between talin, a protein that links liganded membrane integrins to the cytoskeleton, and vinculin, a focal adhesion protein that is activated by talin binding, leading to reorganization of the cytoskeleton. Application of physiologically relevant forces caused stretching of single talin rods that exposed cryptic binding sites for vinculin. Thus in the talin-vinculin system, molecular mechanotransduction can occur by protein binding after exposure of buried binding sites in the talin-vinculin system. Such protein stretching may be a more general mechanism for force transduction.
Figures
Fig. 1.
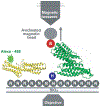
(A) Structure of the 12 helices that form the TR 482 to 889 [Protein Data Bank (PDB) 1xwx]. Color coding: blue, N terminus; green, middle; and red, C terminus. VBS helices are numbered and represented with cartoon models, and the rest of the protein is shown with tube models. (B) Under the application of a force in the direction indicated by the black arrows in (A), TR starts to unfold. Once helix 12 exposed its VBS, Vh (PDB 1u6h), represented in yellow, reorganizes to bind to it. (C) X-ray structure of the complex Vh-VBS-helix 12, PDB 1u6h. Images were generated by using the VMD (Visual Molecular Dynamics) program (
).
Fig. 2.
Representation of the device used to measure the binding events. The Ni-NTA (labeled N) grafted slides containing the TR fixed through its 6×His N terminus (labeled H) to the glass and with the avidinated magnetic bead bound to its biotinylated C terminus (labeled B) was placed over the objective. Alexa 488–Vh was added to the slides for the period of the incubation. The TR and Vh structures are represented in green and yellow, respectively. The arrow shows the direction of the movement of the beads when they are pulled using the magnetic tweezers.
Fig. 3.
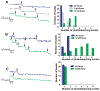
Diagram of photobleaching events of Alexa 488–Vh bound to (A) TR, (B) dimeric tandem TR, and (C) α-actinin. Histograms show the number of beads per photobleaching event. In all cases, blue, gray, and green colors correspond with no force, 2-pN force, and 12-pN force applied, respectively. The TR, talin dimeric tandem (positive control), and α-actinin (negative control) showed maximally 1 and 3, 2 and 6, and 1 and 1 photobleaching events (black arrows) when no force and 12 pN force, respectively, was applied.
Fig. 4.
AFM experiments. (A) Diagram of the polyprotein designed for AFM, I272–TR–I272. (B) Force extension trace for I272-TR-I272. (C) Histogram of the contour length increments (Δ_L_c) for the unfolding peaks of the TR (120 traces). (D) Force-clamp trace obtained by stretching I272-TR-I272 at 20 and 150 pN of force. The red and black lines represent extension and force, respectively. (E) Probability of unfolding versus time for TR. Average recordings of TR unfolding at 20 pN (15 traces), 30 pN (14 traces), 40 pN (17 traces), and 50 pN (22 traces). A single exponential (colored smooth lines) is fitted to each average trace (in black). (F) The rate constant of unfolding as a function of force. Data are represented as mean ± SD; error bars were calculated by using the bootstrapping method (26).
Comment in
- Cell biology. The force is with us.
Schwartz MA. Schwartz MA. Science. 2009 Jan 30;323(5914):588-9. doi: 10.1126/science.1169414. Science. 2009. PMID: 19179515 No abstract available.
Similar articles
- A vinculin binding domain from the talin rod unfolds to form a complex with the vinculin head.
Fillingham I, Gingras AR, Papagrigoriou E, Patel B, Emsley J, Critchley DR, Roberts GC, Barsukov IL. Fillingham I, et al. Structure. 2005 Jan;13(1):65-74. doi: 10.1016/j.str.2004.11.006. Structure. 2005. PMID: 15642262 - Mechanical activation of vinculin binding to talin locks talin in an unfolded conformation.
Yao M, Goult BT, Chen H, Cong P, Sheetz MP, Yan J. Yao M, et al. Sci Rep. 2014 Apr 9;4:4610. doi: 10.1038/srep04610. Sci Rep. 2014. PMID: 24714394 Free PMC article. - Intermolecular versus intramolecular interactions of the vinculin binding site 33 of talin.
Yogesha SD, Sharff A, Bricogne G, Izard T. Yogesha SD, et al. Protein Sci. 2011 Aug;20(8):1471-6. doi: 10.1002/pro.671. Protein Sci. 2011. PMID: 21648001 Free PMC article. - Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion.
Critchley DR. Critchley DR. Biochem Soc Trans. 2004 Nov;32(Pt 5):831-6. doi: 10.1042/BST0320831. Biochem Soc Trans. 2004. PMID: 15494027 Review. - Integrin connections to the cytoskeleton through talin and vinculin.
Ziegler WH, Gingras AR, Critchley DR, Emsley J. Ziegler WH, et al. Biochem Soc Trans. 2008 Apr;36(Pt 2):235-9. doi: 10.1042/BST0360235. Biochem Soc Trans. 2008. PMID: 18363566 Review.
Cited by
- Roles of the Dbl family of RhoGEFs in mechanotransduction - a review.
Ohashi K, Kunitomi A, Chiba S, Mizuno K. Ohashi K, et al. Front Cell Dev Biol. 2024 Oct 16;12:1485725. doi: 10.3389/fcell.2024.1485725. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39479515 Free PMC article. Review. - Integrin inactivators: balancing cellular functions in vitro and in vivo.
Bouvard D, Pouwels J, De Franceschi N, Ivaska J. Bouvard D, et al. Nat Rev Mol Cell Biol. 2013 Jul;14(7):430-42. doi: 10.1038/nrm3599. Epub 2013 May 30. Nat Rev Mol Cell Biol. 2013. PMID: 23719537 Review. - Pre-complexation of talin and vinculin without tension is required for efficient nascent adhesion maturation.
Han SJ, Azarova EV, Whitewood AJ, Bachir A, Guttierrez E, Groisman A, Horwitz AR, Goult BT, Dean KM, Danuser G. Han SJ, et al. Elife. 2021 Mar 30;10:e66151. doi: 10.7554/eLife.66151. Elife. 2021. PMID: 33783351 Free PMC article. - The interplay of membrane cholesterol and substrate on vascular smooth muscle biomechanics.
Sanyour HJ, Rickel AP, Hong Z. Sanyour HJ, et al. Curr Top Membr. 2020;86:279-299. doi: 10.1016/bs.ctm.2020.08.003. Epub 2020 Sep 28. Curr Top Membr. 2020. PMID: 33837696 Free PMC article. - Construction, imaging, and analysis of FRET-based tension sensors in living cells.
LaCroix AS, Rothenberg KE, Berginski ME, Urs AN, Hoffman BD. LaCroix AS, et al. Methods Cell Biol. 2015;125:161-86. doi: 10.1016/bs.mcb.2014.10.033. Epub 2015 Jan 8. Methods Cell Biol. 2015. PMID: 25640429 Free PMC article.
References
- Vogel V, Sheetz M, Nat. Rev. Mol. Cell Biol 7, 265 (2006). - PubMed
- Critchley DR, Biochem. Soc. Trans 33, 1308 (2005). - PubMed
- Critchley DR, Curr. Opin. Cell Biol 12, 133 (2000). - PubMed
- Izard T, Vonrhein C, J. Biol. Chem 279, 27667 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases