Knocking down barriers: advances in siRNA delivery - PubMed (original) (raw)
Review
Knocking down barriers: advances in siRNA delivery
Kathryn A Whitehead et al. Nat Rev Drug Discov. 2009 Feb.
Erratum in
- Nat Rev Drug Discov. 2009 Jun;8(6):516
Abstract
In the 10 years that have passed since the Nobel prize-winning discovery of RNA interference (RNAi), billions of dollars have been invested in the therapeutic application of gene silencing in humans. Today, there are promising data from ongoing clinical trials for the treatment of age-related macular degeneration and respiratory syncytial virus. Despite these early successes, however, the widespread use of RNAi therapeutics for disease prevention and treatment requires the development of clinically suitable, safe and effective drug delivery vehicles. Here, we provide an update on the progress of RNAi therapeutics and highlight novel synthetic materials for the encapsulation and intracellular delivery of nucleic acids.
Figures
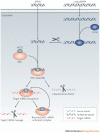
Figure 1. The mechanism of RNA interference.
Long double-stranded RNA (dsRNA) is introduced into the cytoplasm, where it is cleaved into small interfering RNA (siRNA) by the enzyme Dicer. Alternatively, siRNA can be introduced directly into the cell. The siRNA is then incorporated into the RNA-induced silencing complex (RISC), resulting in the cleavage of the sense strand of RNA by argonaute 2 (AGO2). The activated RISC–siRNA complex seeks out, binds to and degrades complementary mRNA, which leads to the silencing of the target gene. The activated RISC–siRNA complex can then be recycled for the destruction of identical mRNA targets.
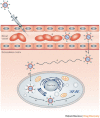
Figure 2. Physiological barriers to the systemic delivery of small interfering RNA (siRNA) nanoparticles.
An injected nanoparticle must avoid filtration, phagocytosis and degradation in the bloodstream (a); be transported across the vascular endothelial barrier (b); diffuse through the extracellular matrix (c); be taken up into the cell (d); escape the endosome (e); and unpackage and release the siRNA to the RNA interference (RNAi) machinery (f).
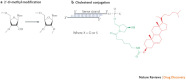
Figure 3. Two common small interfering RNA (siRNA) modifications used for therapeutic applications.
a | The 2′-_O_-methyl sugar modification prevents activation of the Toll-like receptor 7 immune response and confers enzymatic resistance to the siRNA molecule. b | The conjugation of cholesterol to the sense strand of an siRNA duplex improves delivery of naked siRNA to certain cellular targets, including hepatocytes. The cholesterol conjugate is shown in red, and the linker is shown in green.
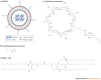
Figure 4. Synthetic materials for small interfering RNA (siRNA) delivery.
a | A representation of stable nucleic acid–lipid particles (SNALPs), which are liposomes comprising cationic lipids, non-ionic lipids and polyethylene glycol (PEG). siRNA is contained in the hydrophilic interior of the particle. b | Polyethyleneimine is used to fabricate both linear and branched polymeric delivery agents. c | A cyclodextrin-based delivery agent. d | The lipidoid 98N12-5(1).
Similar articles
- Recent advances in siRNA delivery.
Sarisozen C, Salzano G, Torchilin VP. Sarisozen C, et al. Biomol Concepts. 2015 Dec;6(5-6):321-41. doi: 10.1515/bmc-2015-0019. Biomol Concepts. 2015. PMID: 26609865 Review. - Biomaterials in siRNA Delivery: A Comprehensive Review.
Ho W, Zhang XQ, Xu X. Ho W, et al. Adv Healthc Mater. 2016 Nov;5(21):2715-2731. doi: 10.1002/adhm.201600418. Epub 2016 Oct 4. Adv Healthc Mater. 2016. PMID: 27700013 Review. - Harnessing RNA interference to develop neonatal therapies: from Nobel Prize winning discovery to proof of concept clinical trials.
DeVincenzo JP. DeVincenzo JP. Early Hum Dev. 2009 Oct;85(10 Suppl):S31-5. doi: 10.1016/j.earlhumdev.2009.08.013. Epub 2009 Oct 14. Early Hum Dev. 2009. PMID: 19833462 - Powering up the molecular therapy of RNA interference by novel nanoparticles.
Liao W, Li W, Zhang T, Kirberger M, Liu J, Wang P, Chen W, Wang Y. Liao W, et al. Biomater Sci. 2016 Jun 21;4(7):1051-61. doi: 10.1039/c6bm00204h. Biomater Sci. 2016. PMID: 27221980 Review. - Polycation-based nanoparticle delivery for improved RNA interference therapeutics.
Howard KA, Kjems J. Howard KA, et al. Expert Opin Biol Ther. 2007 Dec;7(12):1811-22. doi: 10.1517/14712598.7.12.1811. Expert Opin Biol Ther. 2007. PMID: 18034647 Review.
Cited by
- Role of the IGF-1 Axis in Overcoming Resistance in Breast Cancer.
Ianza A, Sirico M, Bernocchi O, Generali D. Ianza A, et al. Front Cell Dev Biol. 2021 Mar 22;9:641449. doi: 10.3389/fcell.2021.641449. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33829018 Free PMC article. Review. - miRNA-Based Therapeutic Strategies.
Ishida M, Selaru FM. Ishida M, et al. Curr Anesthesiol Rep. 2013 Mar 1;1(1):63-70. doi: 10.1007/s40139-012-0004-5. Epub 2012 Dec 24. Curr Anesthesiol Rep. 2013. PMID: 23524956 Free PMC article. - Lipopolymer/siRNA Nanoparticles Targeting the Signal Transducer and Activator of Transcription 5A Disrupts Proliferation of Acute Lymphoblastic Leukemia.
Nasrullah M, Kc R, Nickel K, Parent K, Kucharski C, Meenakshi Sundaram DN, Rajendran AP, Jiang X, Brandwein J, Uludağ H. Nasrullah M, et al. ACS Pharmacol Transl Sci. 2024 Aug 15;7(9):2840-2855. doi: 10.1021/acsptsci.4c00336. eCollection 2024 Sep 13. ACS Pharmacol Transl Sci. 2024. PMID: 39296267 - Role of circular RNAs and gut microbiome in gastrointestinal cancers and therapeutic targets.
Abdullah ST, Abdullah SR, Hussen BM, Younis YM, Rasul MF, Taheri M. Abdullah ST, et al. Noncoding RNA Res. 2023 Dec 15;9(1):236-252. doi: 10.1016/j.ncrna.2023.12.002. eCollection 2024 Mar. Noncoding RNA Res. 2023. PMID: 38192436 Free PMC article. Review. - A physical model for the size-dependent cellular uptake of nanoparticles modified with cationic surfactants.
Xu A, Yao M, Xu G, Ying J, Ma W, Li B, Jin Y. Xu A, et al. Int J Nanomedicine. 2012;7:3547-54. doi: 10.2147/IJN.S32188. Epub 2012 Jul 10. Int J Nanomedicine. 2012. PMID: 22848178 Free PMC article.
References
- Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
- Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. - PubMed
- McCaffrey AP, et al. Gene expression: RNA interference in adult mice. Nature. 2002;418:38–39. - PubMed
- Morrissey DV, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nature Biotech. 2005;23:1002–1007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources