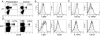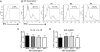Cytokine-induced memory-like natural killer cells - PubMed (original) (raw)
Cytokine-induced memory-like natural killer cells
Megan A Cooper et al. Proc Natl Acad Sci U S A. 2009.
Abstract
The mammalian immune response to infection is mediated by 2 broad arms, the innate and adaptive immune systems. Innate immune cells are a first-line defense against pathogens and are thought to respond consistently to infection, regardless of previous exposure, i.e., they do not exhibit memory of prior activation. By contrast, adaptive immune cells display immunologic memory that has 2 basic characteristics, antigen specificity and an amplified response upon subsequent exposure. Whereas adaptive immune cells have rearranged receptor genes to recognize the universe of antigens, natural killer (NK) cells are innate immune lymphocytes with a limited repertoire of germ-line encoded receptors for target recognition. NK cells also produce cytokines such as IFN-gamma (IFN-gamma) to protect the host during the innate response to infection. Herein, we show that cytokine-activated NK cells transferred into naïve hosts can be specifically detected 7-22 days later when they are phenotypically similar to naïve cells and are not constitutively producing IFN-gamma. However, they produce significantly more IFN-gamma when restimulated. This memory-like property is intrinsic to the NK cell. By contrast, memory-like NK cells do not express granzyme B protein and kill targets similarly to naïve NK cells. Thus, these experiments identify an ability of innate immune cells to retain an intrinsic memory of prior activation, a function until now attributed only to antigen-specific adaptive immune cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Adoptively transferred, preactivated, and control NK cells can be detected after 1–3 weeks and are phenotypically similar. (A) CFSE-labeled preactivated (IL-12 + IL-18 with 10 ng/mL IL-15) or control-treated (10 ng/mL IL-15) NK cells were transferred into _Rag1_−/− hosts and could be detected 7 (Upper) to 22 (Lower) days later in the spleen, identified as CFSE+NK1.1+. Percentages represent transferred NK cells, gated on live lymphocytes. (B) Preactivated (solid black line) and control (dashed line) NK cells express similar levels of NK activation markers (CD69 and CD11b), CD27, and B220. (C) Cytokine receptor expression 7 days after adoptive transfer (isotype control, shaded). Results are representative of 2–5 independent experiments.
Fig. 2.
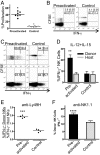
Preactivated NK cells proliferate in vivo and do not constitutively make IFN-γ but produce abundant IFN-γ upon restimulation. (A) NK cells preactivated with IL-12 + IL-18 (plus 10 ng/mL IL-15) proliferate in vivo significantly more than control-treated (10 ng/mL IL-15) cells (40.5% v. 6.3%;*, P < 0.0001; n = 7). (B) Preactivated (Left) donor-derived (CFSE+) and host (CFSE−) NK cells do not constitutively produce IFN-γ 7 days after adoptive transfer. (C) Representative FACS plot of CFSE+ donor NK cell (Upper) and CFSE− host NK cell (Lower) IFN-γ production (x axis) after restimulation with IL-12 + IL-15 for 4 h. All plots are gated on NK1.1+ NK cells. The numbers indicate the percentage of CSFE+ (donor) cells (Upper) or CSFE− (host) cells (Lower) in the corresponding gates, demonstrating significantly more preactivated NK cells produce IFN-γ upon restimulation. (D) Percentage of IFN-γ positive CFSE+ (donor) and CFSE− (host) NK cells after stimulation with IL-12 + IL-15 (**, P < 0.01, n = 4). (E and F) Engagement of Ly49H (E) or NK1.1 (F) by culture with plate-bound monoclonal antibody for 8 h (***, P < 0.0001; ****, P = 0.004). IFN-γ production with anti-Ly49H is shown as a ratio of % IFN-γ positive donor NK cells (CFSE+NK1.1+) to % IFN-γ positive host NK cells (CFSE−NK1.1+) to account for well-to-well variability. Error bars indicate SEM.
Fig. 3.
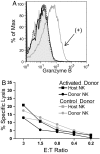
Memory-like NK cells lack granzyme B protein and kill target cells similar to control NK cells. (A) Preactivated, memory-like NK cells (solid black line) do not express granzyme B protein 7 days after adoptive transfer into _Rag1_−/− hosts as compared with a positive control, NK cells activated with high-dose (100 ng/mL) IL-15 for 2 days. (Dashed line, control-treated NK cells; shaded, negative control; gray line, positive control). (B) Donor and host NK cells were sorted from recipients that had received activated or control NK cells 7 days prior and were used as effectors in a cytotoxicity assay with Yac-1 targets at the indicated effector to target (E:T) ratios. Data represent the mean of duplicate wells and results are representative of 2 independent experiments.
Fig. 4.
Similar IFN-γ production by memory-like NK cells regardless of proliferation. (A) Resting NK cells did not produce IFN-γ protein. (B) After restimulation with IL-12 + IL-15, parental (generation 0) and 3 daughter generations of preactivated memory-like NK cells were identified based on CFSE dilution and IFN-γ production in each generation measured 7 days after adoptive transfer. (C and D) There was no significant difference in IFN-γ production in generations 0–3 after restimulation with either IL-12 + IL-15 (C) or anti-Ly49H plate-bound antibody (D). Results represent the mean of 3 independent experiments. Error bars indicate SEM.
Similar articles
- Memory-like responses of natural killer cells.
Cooper MA, Yokoyama WM. Cooper MA, et al. Immunol Rev. 2010 May;235(1):297-305. doi: 10.1111/j.0105-2896.2010.00891.x. Immunol Rev. 2010. PMID: 20536571 Free PMC article. Review. - Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation.
Keppel MP, Yang L, Cooper MA. Keppel MP, et al. J Immunol. 2013 May 1;190(9):4754-62. doi: 10.4049/jimmunol.1201742. Epub 2013 Mar 25. J Immunol. 2013. PMID: 23530145 Free PMC article. - Dendritic cells and NK cells stimulate bystander T cell activation in response to TLR agonists through secretion of IFN-alpha beta and IFN-gamma.
Kamath AT, Sheasby CE, Tough DF. Kamath AT, et al. J Immunol. 2005 Jan 15;174(2):767-76. doi: 10.4049/jimmunol.174.2.767. J Immunol. 2005. PMID: 15634897 - [Natural killer cells: adaptation and memory in innate immunity].
Narni-Mancinelli E, Ugolini S, Vivier E. Narni-Mancinelli E, et al. Med Sci (Paris). 2013 Apr;29(4):389-95. doi: 10.1051/medsci/2013294012. Epub 2013 Apr 26. Med Sci (Paris). 2013. PMID: 23621934 Review. French. - Relative contributions of NK and CD8 T cells to IFN-gamma mediated innate immune protection against Listeria monocytogenes.
Berg RE, Crossley E, Murray S, Forman J. Berg RE, et al. J Immunol. 2005 Aug 1;175(3):1751-7. doi: 10.4049/jimmunol.175.3.1751. J Immunol. 2005. PMID: 16034116 Free PMC article.
Cited by
- Natural killer cell therapies.
Vivier E, Rebuffet L, Narni-Mancinelli E, Cornen S, Igarashi RY, Fantin VR. Vivier E, et al. Nature. 2024 Feb;626(8000):727-736. doi: 10.1038/s41586-023-06945-1. Epub 2024 Feb 21. Nature. 2024. PMID: 38383621 Review. - A discrete subset of epigenetically primed human NK cells mediates antigen-specific immune responses.
Stary V, Pandey RV, Strobl J, Kleissl L, Starlinger P, Pereyra D, Weninger W, Fischer GF, Bock C, Farlik M, Stary G. Stary V, et al. Sci Immunol. 2020 Oct 16;5(52):eaba6232. doi: 10.1126/sciimmunol.aba6232. Sci Immunol. 2020. PMID: 33067380 Free PMC article. - Human NK Cell Cytoskeletal Dynamics and Cytotoxicity Are Regulated by LIM Kinase.
Duvall MG, Fuhlbrigge ME, Reilly RB, Walker KH, Kılıç A, Levy BD. Duvall MG, et al. J Immunol. 2020 Aug 1;205(3):801-810. doi: 10.4049/jimmunol.2000186. Epub 2020 Jul 8. J Immunol. 2020. PMID: 32641387 Free PMC article. - Targeting natural killer cells: from basic biology to clinical application in hematologic malignancies.
Shang J, Hu S, Wang X. Shang J, et al. Exp Hematol Oncol. 2024 Feb 23;13(1):21. doi: 10.1186/s40164-024-00481-y. Exp Hematol Oncol. 2024. PMID: 38396050 Free PMC article. Review. - IL-12/15/18-preactivated NK cells suppress GvHD in a mouse model of mismatched hematopoietic cell transplantation.
Hüber CM, Doisne JM, Colucci F. Hüber CM, et al. Eur J Immunol. 2015 Jun;45(6):1727-35. doi: 10.1002/eji.201445200. Epub 2015 Apr 17. Eur J Immunol. 2015. PMID: 25778912 Free PMC article.
References
- Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. - PubMed
- Orange JS. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol. 2006;6:399–409. - PubMed
- Bustamante J, et al. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr Opin Immunol. 2008;20:39–48. - PubMed
- Yokoyama WM. Mistaken notions about natural killer cells. Nat Immunol. 2008;9:481–485. - PubMed
- Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI034385/AI/NIAID NIH HHS/United States
- R01 AI033903/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- AI51345/AI/NIAID NIH HHS/United States
- AI33903/AI/NIAID NIH HHS/United States
- R01 AI051345/AI/NIAID NIH HHS/United States
- T32 HD043010/HD/NICHD NIH HHS/United States
- R01 AI034385/AI/NIAID NIH HHS/United States
- AI34385/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources