Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses - PubMed (original) (raw)
Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses
Ye Zheng et al. Nature. 2009.
Abstract
In the course of infection or autoimmunity, particular transcription factors orchestrate the differentiation of T(H)1, T(H)2 or T(H)17 effector cells, the responses of which are limited by a distinct lineage of suppressive regulatory T cells (T(reg)). T(reg) cell differentiation and function are guided by the transcription factor Foxp3, and their deficiency due to mutations in Foxp3 results in aggressive fatal autoimmune disease associated with sharply augmented T(H)1 and T(H)2 cytokine production. Recent studies suggested that Foxp3 regulates the bulk of the Foxp3-dependent transcriptional program indirectly through a set of transcriptional regulators serving as direct Foxp3 targets. Here we show that in mouse T(reg) cells, high amounts of interferon regulatory factor-4 (IRF4), a transcription factor essential for T(H)2 effector cell differentiation, is dependent on Foxp3 expression. We proposed that IRF4 expression endows T(reg) cells with the ability to suppress T(H)2 responses. Indeed, ablation of a conditional Irf4 allele in T(reg) cells resulted in selective dysregulation of T(H)2 responses, IL4-dependent immunoglobulin isotype production, and tissue lesions with pronounced plasma cell infiltration, in contrast to the mononuclear-cell-dominated pathology typical of mice lacking T(reg) cells. Our results indicate that T(reg) cells use components of the transcriptional machinery, promoting a particular type of effector CD4(+) T cell differentiation, to efficiently restrain the corresponding type of the immune response.
Figures
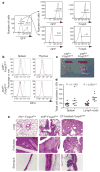
Figure 1. Ablation of IRF4 in Treg cells results in autoimmune lymphoproliferative disease
a, _Foxp3Cre_-mediated deletion of Irf4 is restricted to Treg cells. Spleen and lymph node CD4+ T cells from _Irf4fl/− Foxp3Cre mice were FACS-sorted into GFP+ and GFP− cells containing a recombined and unrecombined Irf4fl allele, respectively, and stained for Foxp3. The post-sorting purity of GFP+ and GFP− population was greater than 97%. b, Intracellular staining of IRF4 in splenic and thymic CD4+ Foxp3− (red) and CD4+ Foxp3+ (blue) cells and splenic B220− CD138+ (green) plasma cells (positive control) in Irf4fl/+ Foxp3Cre (top panels) and Irf4fl/flFoxp3Cre mice (bottom panels). c, Splenomegaly and lymphadenopathy in Irf4fl/flFoxp3Cre mice. d, Spleen and lymph node cellularity in Irf4fl/+ Foxp3Cre (filled circles) and Irf4fl/flFoxp3Cre (open circles) mice. e, Histopathology induced after IRF4 ablation in Treg cells. Representative haematoxylin and eosin (H&E)-stained tissue sections from 8-week-old Irf4+/_− Foxp3Cre and Irf4fl/-Foxp3Cre mice and diphtheria-toxin-treated Foxp3DTR mice. Note pronounced infiltrates in the lung, pancreas and stomach of _Irf4fl/_− Foxp3Cre mice and severe lesions in tissues from diphtheria-toxin-treated Foxp3DTR mice (n = 3–5). Original magnification (c, e), ×10.
Figure 2. Increased numbers and activation of CD4+ T cells in mice harbouring IRF4-deficient Tregcells
a, CD4+ and CD8+ T cell numbers in the spleen (sp) and lymph nodes (ln) of Irf4fl/flFoxp3Cre (open circles) mice and Irf4fl/+ Foxp3Cre (filled circles) littermate control mice. NS, not significant. b, Flow cytometric analysis of CD44, CD62L and CD69 expression on CD4+ T cells in 8-week-old Irf4fl/flFoxp3Cre mice and Irf4fl/+ Foxp3Cre littermates. A representative of three independent experiments is shown. c, d, Increased Foxp3+ Treg cell subset in Irf4fl/flFoxp3Cre mice. Flow cytometric analyses of spleen and lymph node cells from Irf4fl/+ Foxp3Cre (filled circles) and Irf4fl/flFoxp3Cre (open circles) mice. A representative of three independent experiments is shown.
Figure 3. IRF4 deficiency in Treg cells results in a selective failure to control TH2 responses
a, b, Flow cytometric analysis of cytokine production by splenic CD4+ T cells from Irf4fl/+ Foxp3Cre mice and Irf4fl/flFoxp3Cre littermates. Splenocytes were stimulated with CD3 (5 μg ml−1) and CD28 (5 μg ml-1) antibodies in the presence of Golgi-Plug (1 μg ml−1) for 5 h before staining for CD4, CD8 and the indicated cytokines. A representative of three independent experiments is shown.
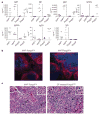
Figure 4. Increased serum IgG1 and IgE concentration, germinal centre formation, and plasma cell tissue infiltration caused by IRF4 deficiency in Treg cells
a, Analysis of immunoglobulin isotype amounts in sera of 8-week-old Irf4fl/flFoxp3Cre mice and Irf4fl/+ Foxp3Cre littermates, and of 3–4-week-old _Foxp3_− and Foxp3+ littermates. b, Immunofluorescent staining of germinal centre B cells (GL7+, green), follicular B cells (IgD+, red), and CD4+ T cells (blue) in spleens of Irf4fl/flFoxp3Cre mice and Irf4fl/+ Foxp3Cre littermates. Original magnification, ×20. c, Histological sections of H&E-stained pancreas from 8-week-old _Irf4fl_− Foxp3Cre mice and Treg-deficient diphtheria-toxin-treated Foxp3DTR mice. The _Irf4fl/_− Foxp3Cre pancreas is infiltrated primarily by plasma cells (arrows), that is, distinct round cells containing an eccentric nucleus with a cartwheel chromatin appearance and perinuclear clearing (inset; original magnification, ×60). In contrast, the pancreatic infiltrates of diphtheria-toxin-treated Foxp3DTR mice contained principally macrophages (arrows), that is, large cells with abundant eosinophilic cytoplasm, reniform to oval nuclei, and indistinct cell borders (inset; original magnification, ×60). Original magnification for both panels, ×40. Representative sections are shown.
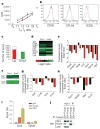
Figure 5. IRF4 interacts with Foxp3 and diminished expression of a subset of suppressor effector and TH2 specific genes in IRF4-deficient Treg cells
a, IRF4-sufficient (Irf4+ TR, red) and -deficient (_Irf4_− TR, blue) Treg cells from 5-week-old Irf4+/+ Foxp3Cre mice and Irf4fl/flFoxp3Cre littermates suppress in vitro proliferative response of Foxp3− CD4+ T cells (TE) from B6 mice. A representative of two independent experiments is shown. b, Flow cytometric analysis of CD25, CTLA4 and ICOS expression by IRF4-sufficent (red) and -deficient (blue) Treg cells. c, Numbers of IRF4-independent genes that were up- (red bars) or downregulated (green bars), respectively, in Treg cells compared to naive CD25− Foxp3− CD4+ T cells. Open bars represent genes in which expression was changed by twofold or more in the absence of IRF4. d, Decreased expression of genes with a presumed role in Treg suppressor function in IRF4-deficient compared to IRF4-sufficient Treg cells. The data (c, d) represent average of two independent microarray experiments (exp) performed using YFP-Cre+ Treg cells FACS-purified from healthy _Irf4fl/_− Foxp3Cre/wt and Irf4+/− Foxp3Cre littermates. e, qPCR analysis of relative expression of genes shown in d in Irf4+ Treg (black) and _Irf4_− Treg (red). f, The decreased expression of TH2-specific or functionally important genes in IRF4-deficient in comparison to IRF4-sufficient Treg cells (two independent microarray experiments as above). g, h, qPCR analysis of relative expression of the TH2-specific gene set in Irf4+ Treg (black) and _Irf4_− Treg (red) (g), and in in vitro differentiated TH1 (black) and TH2 cells (red) (h). Data in e, g and h represent mean and s.d. of the expression of genes relative to Hprt1 in two independent experiments using three replicates each. i, Both IRF4 and Foxp3 bind to the promoter region of the Icos gene. qPCR analysis of Foxp3- and IRF4-bound chromatin isolated from wild-type Treg cells using primer set corresponding to the Icos promoter region. IgG ChIP and qPCR using primers corresponding to the promoter region of Gmpr was used as a specificity controls. j, Western blot (WB) analysis of IRF4 in nuclear lysates of wild-type Foxp3+ Treg cells and total Foxp3− CD4+ T cells (control) (lanes 1 and 2), and in Foxp3 complexes immunoprecipitated (IP) from the nuclear lysates using Foxp3 antibody. Transcription factor p65 was a negative control. IRF4 signal in control nuclear lysates is due to the presence of activated IRF4+ CD25+ Foxp3− T cells.
Similar articles
- Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression.
Wan YY, Flavell RA. Wan YY, et al. Nature. 2007 Feb 15;445(7129):766-70. doi: 10.1038/nature05479. Epub 2007 Jan 14. Nature. 2007. PMID: 17220876 - Lack of Foxp3 function and expression in the thymic epithelium.
Liston A, Farr AG, Chen Z, Benoist C, Mathis D, Manley NR, Rudensky AY. Liston A, et al. J Exp Med. 2007 Mar 19;204(3):475-80. doi: 10.1084/jem.20062465. Epub 2007 Mar 12. J Exp Med. 2007. PMID: 17353370 Free PMC article. - Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate.
Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Zheng Y, et al. Nature. 2010 Feb 11;463(7282):808-12. doi: 10.1038/nature08750. Epub 2010 Jan 13. Nature. 2010. PMID: 20072126 Free PMC article. - Plasticity of T(reg) cells: is reprogramming of T(reg) cells possible in the presence of FOXP3?
Beyer M, Schultze JL. Beyer M, et al. Int Immunopharmacol. 2011 May;11(5):555-60. doi: 10.1016/j.intimp.2010.11.024. Epub 2010 Nov 27. Int Immunopharmacol. 2011. PMID: 21115121 Review. - Human regulatory T cells: a unique, stable thymic subset or a reversible peripheral state of differentiation?
Pillai V, Karandikar NJ. Pillai V, et al. Immunol Lett. 2007 Nov 30;114(1):9-15. doi: 10.1016/j.imlet.2007.08.012. Epub 2007 Sep 29. Immunol Lett. 2007. PMID: 17945352 Free PMC article. Review.
Cited by
- Cell-based therapies for the treatment of rheumatoid arthritis.
Moghaddam MZ, Mousavi MJ, Ghotloo S. Moghaddam MZ, et al. Immun Inflamm Dis. 2023 Nov;11(11):e1091. doi: 10.1002/iid3.1091. Immun Inflamm Dis. 2023. PMID: 38018576 Free PMC article. Review. - Function and Role of Regulatory T Cells in Rheumatoid Arthritis.
Jiang Q, Yang G, Liu Q, Wang S, Cui D. Jiang Q, et al. Front Immunol. 2021 Apr 1;12:626193. doi: 10.3389/fimmu.2021.626193. eCollection 2021. Front Immunol. 2021. PMID: 33868244 Free PMC article. Review. - Highlights of the advances in basic immunology in 2011.
Liu J, Liu S, Cao X. Liu J, et al. Cell Mol Immunol. 2012 May;9(3):197-207. doi: 10.1038/cmi.2012.12. Epub 2012 Apr 23. Cell Mol Immunol. 2012. PMID: 22522654 Free PMC article. Review. - Mechanisms of regulatory T cell counter-regulation by innate immunity.
Yeh H, Moore DJ, Markmann JF, Kim JI. Yeh H, et al. Transplant Rev (Orlando). 2013 Apr;27(2):61-4. doi: 10.1016/j.trre.2013.02.001. Epub 2013 Mar 7. Transplant Rev (Orlando). 2013. PMID: 23474287 Free PMC article. Review. - Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells.
Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Duhen T, et al. Blood. 2012 May 10;119(19):4430-40. doi: 10.1182/blood-2011-11-392324. Epub 2012 Mar 21. Blood. 2012. PMID: 22438251 Free PMC article.
References
- Ramsdell F. Foxp3 and natural regulatory T cells: key to a cell lineage? Immunity. 2003;19:165–168. - PubMed
- Sakaguchi S, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. - PubMed
- Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nature Immunol. 2007;8:457–462. - PubMed
- Zheng Y, et al. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous