Interaction of the SPG21 protein ACP33/maspardin with the aldehyde dehydrogenase ALDH16A1 - PubMed (original) (raw)
Interaction of the SPG21 protein ACP33/maspardin with the aldehyde dehydrogenase ALDH16A1
Michael C Hanna et al. Neurogenetics. 2009 Jul.
Abstract
Mast syndrome (SPG21) is an autosomal-recessive complicated form of hereditary spastic paraplegia characterized by dementia, thin corpus callosum, white matter abnormalities, and cerebellar and extrapyramidal signs in addition to spastic paraparesis. A nucleotide insertion resulting in premature truncation of the SPG21 gene product acidic cluster protein 33 (ACP33)/maspardin underlies this disorder, likely causing loss of protein function. However, little is known about the function of maspardin. Here, we report that maspardin localizes prominently to cytoplasm as well as to membranes, possibly at trans-Golgi network/late endosomal compartments. Immunoprecipitation of maspardin with identification of coprecipitating proteins by mass spectrometry revealed the aldehyde dehydrogenase ALDH16A1 as an interacting protein. This interaction was confirmed using overexpressed proteins as well as by fusion protein pull down experiments, and these proteins colocalized in cells. Further studies of the function of ALDH16A1 and the role of the maspardin-ALDH16A1 interaction in neuronal cells may clarify the cellular pathogenesis of Mast syndrome.
Figures
Fig. 1
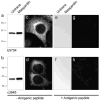
Characterization of polyclonal anti-maspardin antibodies. a, b Lysates from mock-transfected (Untrans; 30 μg protein per lane) or maspardin-transfected (3 μg protein) HeLa cells were immunoblotted with antibodies against N- (a) or C-terminal (b) regions of maspardin. Migration of molecular mass standards (in kilodalton) are shown to the left. c, d HeLa cells were immunostained using N-terminal (c) or C-terminal (d) antibodies, with detection by confocal microscopy. eh Immunodetection for both N- and C-terminal antibodies, respectively, could be eliminated on immunoblots (e, f) and immunostaining (g, h) by preadsorption with the antigenic peptides
Fig. 2
Subcellular distributions of maspardin in HeLa cells. a Fractionation of HeLa cell lysates was performed using the Qiagen Qproteome Cell Compartment Kit. Fractions (20 μg protein per lane) were immunoblotted with antibodies against maspardin (no. c3945), the cytoplasmic protein PLC-γ, the endoplasmic reticulum protein calnexin, and the nuclear envelope protein lamin A + C. Abbreviations: C, cytosolic fraction; M, membrane fraction; N, nuclear fraction. b Subcellular fractions were prepared from HeLa cells by differential centrifugation. Fractions (30 μg of protein per lane) were immunoblotted with antibodies against maspardin, the mitochondrial protein OPA1, calnexin, and PLC-γ. Specific fractions are described in the “Materials and methods.” Abbreviation: H, homogenate. c Detergent-solubilized extracts prepared from HeLa cells were subjected to FPLC gel-exclusion chromatography, and aliquots of collected fractions were immunoblotted with anti-maspardin antibodies. Elution peaks for marker proteins (in kilodalton) are indicated across the top
Fig. 3
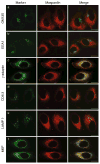
Subcellular localization of maspardin. HeLa cells were coimmunostained for endogenous maspardin (red) and the indicated marker proteins (a_–_f, green). Merged images are at the right. Abbreviation: M6P, man-nose-6-phosphate receptor. Bars, 10 μm
Fig. 4
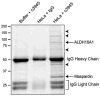
Identification of maspardin-associated proteins in HeLa cells. HeLa cell lysates or else extraction buffer alone was immunoprecipitated using an anti-maspardin antibody (no. c3945), and in control experiments HeLa cells extracts were immunoprecipitated with nonimmune IgG. Proteins coimmunoprecipitating with maspardin were resolved on 10% SDS-PAGE gels and stained with MALDI-TOF-mass-spectroscopy-compatible Coomassie blue. Only proteins present specifically in HeLa + c3945, and not in the nonspecific binding control conditions, were excised for enzymatic digestion by trypsin and identification by mass spectrometry. Maspardin (~33 kDa, arrow) and ALDH16A1 (~80 kDa, arrow) were clearly identified. Other coimmunoprecipitating proteins are evident but have not been independently verified (arrowheads). Heavy and light chains of IgG are indicated
Fig. 5
Maspardin associates with ALDH16A1 in HeLa cells. a, b HeLa cells were mock-transfected or else transfected with full-length ALDH16A1, epitope-tagged as indicated. Extracts were immunoprecipitated with control IgG (−IP) or polyclonal anti-maspardin antibodies to either the N-terminal (b5704) or the C-terminal (c3945) region and then immunoblotted with anti-Myc (a) or anti-HA (b) monoclonal antibodies to detect ALDH16A1. c Reciprocal immunoprecipitation experiments in which endogenous ALDH16A1 was immunoprecipitated using polyclonal anti-ALDH16A1 antibodies and then immunoblotted with anti-Myc antibodies to detect overexpressed Myc-maspardin. d Untransfected HeLa cell lysates or else control lysis buffer were immunoprecipitated with control IgG (−IP) or polyclonal anti-maspardin antibodies and immunoblotted with polyclonal anti-ALDH16A1 antibodies. e Confocal fluorescence microscopy of endogenous ALDH16A1 and maspardin demonstrate similar subcellular localizations within HeLa cells and distinct regions of colocalization (merge). Immuno-reactive signals were greatly reduced by preadsorption of the antibodies with their respective antigenic peptides. Bars, 10 μm
Fig. 6
Specificity of maspardin–ALDH16A1 interactions. a Maspardin interacts with ALDH16A1 in vitro. Immobilized GST–maspardin or control GST were used in pull down assays with purified CBP–ALDH16A1. Bound proteins were resolved by SDS-PAGE and immunoblotted with antibodies against CBP. b Lysates from wild-type Myc-maspardin (WT) or Myc-maspardin-S109A-transfected HeLa cells were immunoprecipitated using anti-Myc antibodies or control mouse IgG and then immunoblotted for ALDH16A1. Endogenous ALDH16A1 is not visible in the input lanes at this exposure but is enriched in anti-Myc immunoprecipitates. c Extracts from untransfected or Myc-ALDH16A1-transfected cells were immunoprecipitated with control IgG or anti-maspardin antibodies (Maspardin IP) and immunoblotted with anti-ALDH1A1 antibodies. In control experiments; protein A–Sepharose beads (Beads) alone were added without immunoglobulins. Migrations of the ALDH1A1 protein and IgG heavy chain are indicated. Migrations of molecular mass standards (in kilodalton) are shown to the right. In all panels, inputs represent 10% of the total lysate
Fig. 7
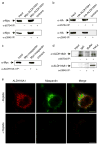
Maspardin and ALDH16A1 interactions in the central nervous system. a RT-PCR reaction products from total RNA extracted from mouse cortex. Sizes are 511 bp for maspardin and 512 bp for ALDH16A1. Full-length cDNA of maspardin and ALDH16A1 serve as both positive and negative controls, as shown. b Untransfected HeLa cell and mouse brain tissue lysates were immunoprecipitated with control IgG (for brain lysates only) or anti-maspardin antibodies and then immunoblotted with anti-ALDH16A1 antibodies. Where indicated (Buffer), no cell extracts were added. c Cultured primary cortical neurons were costained with antibodies against maspardin (green) and ALDH16A1 (red) and imaged using confocal microscopy. Boxed areas in the phase-contrast superimposed image on the left are enlarged on the right
Similar articles
- Maspardin is mutated in mast syndrome, a complicated form of hereditary spastic paraplegia associated with dementia.
Simpson MA, Cross H, Proukakis C, Pryde A, Hershberger R, Chatonnet A, Patton MA, Crosby AH. Simpson MA, et al. Am J Hum Genet. 2003 Nov;73(5):1147-56. doi: 10.1086/379522. Epub 2003 Oct 16. Am J Hum Genet. 2003. PMID: 14564668 Free PMC article. - Targeted disruption of the Mast syndrome gene SPG21 in mice impairs hind limb function and alters axon branching in cultured cortical neurons.
Soderblom C, Stadler J, Jupille H, Blackstone C, Shupliakov O, Hanna MC. Soderblom C, et al. Neurogenetics. 2010 Oct;11(4):369-78. doi: 10.1007/s10048-010-0252-7. Epub 2010 Jul 27. Neurogenetics. 2010. PMID: 20661613 Free PMC article. - Loss of Maspardin Attenuates the Growth and Maturation of Mouse Cortical Neurons.
Davenport A, Bivona A, Latson W, Lemanski LF, Cheriyath V. Davenport A, et al. Neurodegener Dis. 2016;16(3-4):260-72. doi: 10.1159/000443666. Epub 2016 Mar 16. Neurodegener Dis. 2016. PMID: 26978163 - The role of AP-4 in cargo export from the trans-Golgi network and hereditary spastic paraplegia.
Mattera R, De Pace R, Bonifacino JS. Mattera R, et al. Biochem Soc Trans. 2020 Oct 30;48(5):1877-1888. doi: 10.1042/BST20190664. Biochem Soc Trans. 2020. PMID: 33084855 Review. - Hereditary spastic paraplegias with autosomal dominant, recessive, X-linked, or maternal trait of inheritance.
Finsterer J, Löscher W, Quasthoff S, Wanschitz J, Auer-Grumbach M, Stevanin G. Finsterer J, et al. J Neurol Sci. 2012 Jul 15;318(1-2):1-18. doi: 10.1016/j.jns.2012.03.025. Epub 2012 May 1. J Neurol Sci. 2012. PMID: 22554690 Review.
Cited by
- Druggable Targets for Postpartum Depression: A Mendelian Randomization and Colocalization Study.
Wu S, Shen M, Wang H, Zhou W. Wu S, et al. Cell Mol Neurobiol. 2025 Jun 19;45(1):61. doi: 10.1007/s10571-025-01581-x. Cell Mol Neurobiol. 2025. PMID: 40537683 Free PMC article. - Update on the Genetics of Spastic Paraplegias.
Boutry M, Morais S, Stevanin G. Boutry M, et al. Curr Neurol Neurosci Rep. 2019 Feb 28;19(4):18. doi: 10.1007/s11910-019-0930-2. Curr Neurol Neurosci Rep. 2019. PMID: 30820684 Review. - SPG21, a potential oncogene targeted by miR-128-3p, amplifies HBx-induced carcinogenesis and chemoresistance via activation of TRPM7-mediated JNK pathway in hepatocellular carcinoma.
Zhou P, Yao W, Liu L, Yan Q, Chen X, Wei X, Ding S, Lv Z, Zhu F. Zhou P, et al. Cell Oncol (Dordr). 2024 Oct;47(5):1757-1778. doi: 10.1007/s13402-024-00955-5. Epub 2024 May 16. Cell Oncol (Dordr). 2024. PMID: 38753154 - Aldehyde dehydrogenases: from eye crystallins to metabolic disease and cancer stem cells.
Vasiliou V, Thompson DC, Smith C, Fujita M, Chen Y. Vasiliou V, et al. Chem Biol Interact. 2013 Feb 25;202(1-3):2-10. doi: 10.1016/j.cbi.2012.10.026. Epub 2012 Nov 16. Chem Biol Interact. 2013. PMID: 23159885 Free PMC article. Review. - Disease mutations in Rab7 result in unregulated nucleotide exchange and inappropriate activation.
McCray BA, Skordalakes E, Taylor JP. McCray BA, et al. Hum Mol Genet. 2010 Mar 15;19(6):1033-47. doi: 10.1093/hmg/ddp567. Epub 2009 Dec 22. Hum Mol Genet. 2010. PMID: 20028791 Free PMC article.
References
- Schwarz GA, Liu CN. Hereditary (familial) spastic paraplegia: further clinical and pathologic observations. AMA Arch Neurol Psychiatry. 1956;75:144–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases