Correcting aberrant kinetochore microtubule attachments: an Aurora B-centric view - PubMed (original) (raw)
Review
Correcting aberrant kinetochore microtubule attachments: an Aurora B-centric view
Alexander E Kelly et al. Curr Opin Cell Biol. 2009 Feb.
Abstract
The directed movement of chromosomes during mitosis and meiosis relies on microtubule-mediated connections between spindle poles and kinetochores assembled on chromosomes. The molecular basis for the dynamic interaction between microtubules and kinetochores is just beginning to be unveiled. Here, focusing on the mitotic centromere kinase Aurora B, we review our current understanding of the signaling pathways that correct erroneous microtubule attachment at kinetochores. We evaluate several potential models that may explain how maloriented attachments are recognized and processed by the Aurora B pathway.
Figures
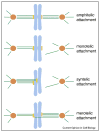
Figure 1
Classification of kinetochore microtubule attachments. Amphitelic attachment: this is the correct attachment, in which all the microtubules attached to a kinetochore connect one spindle pole, while all those attached to its sister kinetochore link to its opposite pole. Monotelic attachment: a kinetochore attaches to microtubules that link to one spindle pole, while its sister kinetochore does not attach to any microtubules. Syntelic attachment: both sister kinetochores are linked to the same pole by microtubules. Merotelic attachment: a kinetochore attaches to microtubules from more than one spindle pole, a situation that results in a lagging chromosome during anaphase. Kinetochores are in yellow, microtubules in green, chromosomes in light blue and centrosomes in orange.
Figure 2
Aurora B pathway control of microtubule attachment. The CPC, composed of Aurora B, INCENP, Dasra (Borealin) and Survivin, is localized to inner centromre, where it signals to correct mal-oriented kinetochore-microtubule attachments. Aurora B can be autoactivated by phosphorylation in trans, but the reaction is inhibited by phosphatases. Upon loss of tension between sister chromatids, inner centromeric Aurora B is activated, where activation may occur directly or indirectly. At the kinetochore, Aurora B phosphorylates Ndc80, leading to destabilization of microtubules at kinetochores. Lack of tension activates the Plk1 dependent phosphorylation of BubR1, a modification which is critical for microtubule attachment [62]. Once microtubule attachment is established, BubR1 is inactivated [63]. Upon bipolar attachment, the Aurora B pathway and the Polo pathway are inactivated, possibly through the action of PP1.
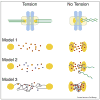
Figure 3
Three models of how the Aurora B pathway is controlled by microtubule attachment or tension. Each model is a simplified enlargement of the inter-kinetochore regions of chromosomes under tension or in a relaxed state. Kinetochores are in orange, microtubules in green, and chromosomes are in light blue. Arrows indicate activation. Model 1: Tension-regulated separation of Aurora B from its kinetochore substrates The distance between active Aurora B at inner centromeres and its kinetochore substrates increases upon bioriented attachment. This increased distance would decrease the likelihood of Aurora B binding kinetochore substrates, which is reinforced by PP1 phosphatase. Model 2: Microtubule-dependent regulation of Aurora B Inner centromeric Aurora B on misaligned chromosomes is more accessible to microtubules than at the metaphase plate. Interactions with microtubules stimulate the kinase activity of Aurora B resulting destabilized microtubule attachments. Model 3: Modulation of Aurora B activity through structural changes in centromeric chromatin Assuming that the CPC is activated by chromatin through a clustering mechanism, the lack of tension may cause a compaction of chromatin at the centromere leading to kinase activation. However, under tension, the chromatin fibers are stretched resulting in a lower effective concentration of the CPC and thus activity. Chromatin fibers are modeled as thin black lines.
Similar articles
- Regulation of kinetochore-microtubule attachments by Aurora B kinase.
Liu D, Lampson MA. Liu D, et al. Biochem Soc Trans. 2009 Oct;37(Pt 5):976-80. doi: 10.1042/BST0370976. Biochem Soc Trans. 2009. PMID: 19754435 Review. - Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity.
Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT. Lan W, et al. Curr Biol. 2004 Feb 17;14(4):273-86. doi: 10.1016/j.cub.2004.01.055. Curr Biol. 2004. PMID: 14972678 - Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates.
Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Liu D, et al. Science. 2009 Mar 6;323(5919):1350-3. doi: 10.1126/science.1167000. Epub 2009 Jan 15. Science. 2009. PMID: 19150808 Free PMC article. - Tension sensing by Aurora B kinase is independent of survivin-based centromere localization.
Campbell CS, Desai A. Campbell CS, et al. Nature. 2013 May 2;497(7447):118-21. doi: 10.1038/nature12057. Epub 2013 Apr 21. Nature. 2013. PMID: 23604256 Free PMC article. - Shake It Off: The Elimination of Erroneous Kinetochore-Microtubule Attachments and Chromosome Oscillation.
Yamamoto A. Yamamoto A. Int J Mol Sci. 2021 Mar 20;22(6):3174. doi: 10.3390/ijms22063174. Int J Mol Sci. 2021. PMID: 33804687 Free PMC article. Review.
Cited by
- A stochastic model of kinetochore-microtubule attachment accurately describes fission yeast chromosome segregation.
Gay G, Courtheoux T, Reyes C, Tournier S, Gachet Y. Gay G, et al. J Cell Biol. 2012 Mar 19;196(6):757-74. doi: 10.1083/jcb.201107124. Epub 2012 Mar 12. J Cell Biol. 2012. PMID: 22412019 Free PMC article. - Aurora-C kinase deficiency causes cytokinesis failure in meiosis I and production of large polyploid oocytes in mice.
Yang KT, Li SK, Chang CC, Tang CJ, Lin YN, Lee SC, Tang TK. Yang KT, et al. Mol Biol Cell. 2010 Jul 15;21(14):2371-83. doi: 10.1091/mbc.e10-02-0170. Epub 2010 May 19. Mol Biol Cell. 2010. PMID: 20484572 Free PMC article. - Cdk1 phosphorylation of the kinetochore protein Nsk1 prevents error-prone chromosome segregation.
Chen JS, Lu LX, Ohi MD, Creamer KM, English C, Partridge JF, Ohi R, Gould KL. Chen JS, et al. J Cell Biol. 2011 Nov 14;195(4):583-93. doi: 10.1083/jcb.201105074. Epub 2011 Nov 7. J Cell Biol. 2011. PMID: 22065639 Free PMC article. - Dynamical scenarios for chromosome bi-orientation.
Zhang T, Oliveira RA, Schmierer B, Novák B. Zhang T, et al. Biophys J. 2013 Jun 18;104(12):2595-606. doi: 10.1016/j.bpj.2013.05.005. Biophys J. 2013. PMID: 23790367 Free PMC article. - The role of Aurora B expression in non-tumor liver tissues of patients with hepatocellular carcinoma.
Tovuu LO, Utsunomiya T, Imura S, Morine Y, Ikemoto T, Arakawa Y, Mori H, Hanaoka J, Kanamoto M, Sugimoto K, Saito Y, Yamada S, Asanoma M, Shimada M. Tovuu LO, et al. Int J Clin Oncol. 2014 Aug;19(4):622-8. doi: 10.1007/s10147-013-0593-y. Epub 2013 Jul 27. Int J Clin Oncol. 2014. PMID: 23893130
References
- Indjeian VB, Murray AW. Budding yeast mitotic chromosomes have an intrinsic bias to biorient on the spindle. Curr Biol. 2007;17:1837–1846. The authors demonstrate that budding yeast Sgo1 is not only required for the tension-sensitive SAC, but also for correction of erroneous kinetochore attachments. Since bipolar attachment is more severely affected in sgo1 mutants with unseparated spindle pole bodies (SPBs) than those with separated SPBs, the authors conclude that yeast chromosomes have an intrinsic geometrical bias to bi-orientation. - PubMed
- Tanaka TU. Bi-orienting chromosomes: acrobatics on the mitotic spindle. Chromosoma. 2008 - PubMed
- Pinsky BA, Biggins S. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 2005;15:486–493. - PubMed
- Dewar H, Tanaka K, Nasmyth K, Tanaka TU. Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle. Nature. 2004 - PubMed
- King JM, Nicklas RB. Tension on chromosomes increases the number of kinetochore microtubules but only within limits. Journal of Cell Science. 2000;113(Pt 21):3815–3823. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources