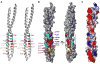Structural basis for recognition of diubiquitins by NEMO - PubMed (original) (raw)
Structural basis for recognition of diubiquitins by NEMO
Yu-Chih Lo et al. Mol Cell. 2009.
Abstract
NEMO is the regulatory subunit of the IkappaB kinase (IKK) in NF-kappaB activation, and its CC2-LZ region interacts with Lys63 (K63)-linked polyubiquitin to recruit IKK to receptor signaling complexes. In vitro, CC2-LZ also interacts with tandem diubiquitin. Here we report the crystal structure of CC2-LZ with two dimeric coiled coils representing CC2 and LZ, respectively. Surprisingly, mutagenesis and nuclear magnetic resonance experiments reveal that the binding sites for diubiquitins at LZ are composites of both chains and that each ubiquitin in diubiquitins interacts with symmetrical NEMO asymmetrically. For tandem diubiquitin, the first ubiquitin uses the conserved hydrophobic patch and the C-terminal tail, while the second ubiquitin uses an adjacent surface patch. For K63-linked diubiquitin, the proximal ubiquitin uses its conserved hydrophobic patch, while the distal ubiquitin mostly employs the C-terminal arm including the K63 linkage residue. These studies uncover the energetics and geometry for mutual recognition of NEMO and diubiquitins.
Figures
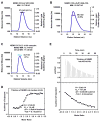
Figure 1
CC2-LZ of NEMO is dimeric and interacts with di-Ub at 2:1 stoichiometry (A) Multi-angle light scattering (MALS) measurement of NEMO CC2-LZ (251–350), showing the relative light scattering signal as a function of elution volume. The derived molecular mass of the peak is shown in violet that matches the reading on the left axis. (B) MALS measurement of NEMO CC2-LZ-ZF (246–419). The derived molecular mass of the peak is shown in pink. (C) MALS measurement of the complex of NEMO CC2-LZ (251–350) and tandem di-Ub. The derived molecular mass of the peak is shown in cyan. (D) Isothermal titration calorimetry (ITC) measurement of the NEMO: tandem di-Ub interaction. NEMO was titrated into the tandem di-Ub solution. The numbers show the fitting of the data, with N for stoichiometry, KD for dissociation constant, ΔH for enthalpy change and -TΔS for entropy change. (E) ITC measurement of the NEMO: K63-di-Ub interaction. NEMO was titrated into the K63-di-Ub solution.
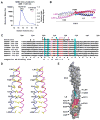
Figure 2
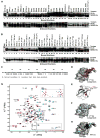
Structure of CC2-LZ (A) MALS measurement of NEMO CC2-LZ (246–337). The major peak (shown in green) corresponds to monomer-dimer equilibrium. (B) Overall structure of CC2-LZ. The N- and C-terminal residues and P299 are labeled. Approximate two-fold axes of the coiled coils are shown by dotted lines. There is a 20° kink between the CC2 and the LZ coiled coils. The overall dimension of the structure is shown. (C) Sequence alignment of CC2-LZ from different species of NEMO and from ABIN1-3 and Optineurin. Mutations of NEMO are shown above the sequence: red, cyan and black indicate drastic, weak and no effects, respectively. Two slight differences between tandem and K63-linked di-Ub were observed. While R319Q had a weak effect on tandem di-Ub interaction, it had no effect on K63-di-Ub interaction. L329P had no effect on tandem di-Ub interaction but had a weak effect on K63-di-Ub interaction. Known disease mutations of NEMO are underlined. C347 is shown in blue. The a and d positions in the coiled coils are labeled below the sequence. Residues important for Ub binding are labeled “1” or “2” depending from which chain they come from in the composite Ub binding site. Part of the sequence of CC2 is omitted in this alignment because there are no absolutely conserved residues in that region. h: human; m: mouse; f: frog; zf: zebrafish. (D) Stereo diagram of the LZ region showing the side chains at the a and d positions of the coiled coil. (E) Mapping of strictly conserved residues onto the CC2-LZ structure. Each residue is shown with a distinct color that matches the colors for mutational effects in Figure 4.
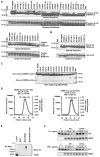
Figure 3
Structure-based mutagenesis of NEMO (A) Pull-down of non-tagged tandem di-Ub by WT and mutant CC2-LZ (residues 251-350). -: essentially no binding; w: weak binding. (B) Pull-down of K63-linked di-Ub by WT and mutant CC2-LZ (residues 251-350). -: essentially no binding; w: weak binding. (C) Non-reducing SDS-PAGE of CC2-LZ mutants that are defective in di-Ub interaction, showing their dimeric state. (D) MALS measurements of selective CC2-LZ mutants, showing their dimeric state. (E) The E315A mutant is defective in interacting with K63-polyubiquitinated IRAK1 in IL-1 signaling. Cell lysates from 293 cells stably expressing IL-1R that had been stimulated with or without IL-1 (10 ng/ml) for 5 min were incubated with GST, GST-NEMO, or GST-NEMO E315A mutant-coated beads and the bead-bound complexes were immunoblotted (IB) with anti-IRAK-1. (F) The E315A mutant is defective in rescuing NEMO-deficient cells in IL-1 and TNFα-induced IκB degradation. Cell lysates from NEMO-deficient MEFs or NEMO-deficient MEFs stably expressing HA-tagged NEMO (WT) or HA-tagged NEMO E315A mutant that had been stimulated with the indicated amounts of IL-1 or TNFα for 10 min were immunoblotted with anti-IκB and anti-NEMO.
Figure 4
Mapped Ub-binding site of NEMO (A) NEMO residues that are important for tandem and K63-linked di-Ub interaction are shown in the stereo ribbon diagram of the CC2-LZ structure. Residues labeled in the red shades caused most drastic effects when mutated. Those labeled in the cyan shades caused less drastic effects. R319 is shown in yellow to indicate that it is involved in tandem, but not K63-linked, di-Ub binding. (B) The same residues are shown on a surface representation of the structure. Residues from one chain are labeled on the right and residues from the other chain are labeled on the left of the structure. The ovals at the right panel indicate the two separate symmetrical Ub-binding sites. (C) The electrostatic surface representation of CC2-LZ.
Figure 5
Structure-based mutagenesis and NMR experiments on the NEMO: tandem di-Ub interaction (A) Mutations on tandem di-Ub. Both Ub molecules are mutated except when noted. R74G/A means R74G in the first Ub and R74A in the second Ub of the tandem di-Ub. The bound di-Ub amounts were semi-quantified. No effects: >0.8 of WT; w+: 0.5–0.8 of WT; w−: 0.2–0.5 of WT; −: <0.2 of WT. (B) Mutations on either the first or the second Ub of the tandem di-Ub. No effects: >0.8 of WT; w+: 0.5–0.8 of WT; w−: 0.2–0.5 of WT; −: <0.2 of WT. (C) Summary of the Ub residues that have been mutated. (D) Mapping of mutational data onto the first Ub surface of the tandem di-Ub. Red: drastic effects; pink: less drastic effects. (E) Mapping of mutational data of both the first and the second Ub onto the ribbon diagram of Ub. Red and pink: residues of the first Ub or both Ub molecules involved in NEMO interaction; cyan and light cyan: residues of the second Ub involved in NEMO interaction. The two ovals indicate approximate locations of the two NEMO-binding sites. (F) Mapping of mutational data onto the second Ub surface of the tandem di-Ub. Cyan: drastic effects; light cyan: less drastic effects. (G)Overlaid contour plots of the 1H-15N HSQC spectra of tandem di-Ub titrated with unlabeled NEMO at different molar ratios: 1:0 (free di-Ub, black), 1:0.1 (red), 1:0.15 (blue), 1:0.2 (green), 1:0.25 (purple) and 1:0.3 (cyan). Side chain peaks are contained in the dashed box in the spectra. (H) Mapping of residues that suffered severe intensity attenuation upon NEMO titration. Green: residues that appear to be selectively attenuated in one of the Ub molecules; blue: residues that appear to be attenuated in both Ub molecules. The ovals indicate the two potential separate NEMO-binding sites. S57 is right behind its labeled position. Residues not shown are buried.
Figure 6
Structure-based mutagenesis and NMR experiments on the NEMO: K63-linked di-Ub interaction (A) Mutations on K63-linked di-Ub. No effects: >0.8 of WT; w+: 0.5–0.8 of WT; w−: 0.2–0.5 of WT; −: <0.2 of WT. (B) Overlaid contour plots of the 1H-15N HSQC spectra of K63-linked di-Ub selectively labeled at the distal Ub and titrated with unlabeled NEMO at different molar ratios: 1:0 (free di-Ub, black), 1:0.1 (red), 1:0.2 (blue), 1:0.3 (green), 1:0.4 (purple), 1:0.5 (cyan) and 1:0.75 (orange). Side chain peaks are contained in the dashed box in the spectra. (C) Overlaid contour plots of the 1H-15N HSQC spectra of K63-linked di-Ub selectively labeled at the proximal Ub and titrated with unlabeled NEMO at different molar ratios: 1:0 (free di-Ub, black), 1:0.1 (red), 1:0.2 (blue), 1:0.3 (green), 1:0.4 (purple), 1:0.5 (cyan) and 1:0.75 (orange). Side chain peaks are contained in the dashed box in the spectra. (D) Chemical shift perturbation (sqrt[(Δδ1H)2 + (Δδ15N)2/25]/2) of distal (solid black bars) and proximal (open red bars) Ub residues upon titration with NEMO. Solid orange and open green bars near * indicate broadened peaks in distal and proximal Ub molecules, respectively. (E) Mapping of residues (cyan) with larger than 0.02ppm chemical shift perturbation (left) and residues with mutational effects (right) onto the surface of distal Ub. For mutational effects, cyan indicates drastic effects and light cyan indicates weak effects. No residues are mapped to the reverse side of Ub. (F) Mapping of residues (red) with larger than 0.02ppm chemical shift perturbation (left) and residues with mutational effects (right) onto the surface of proximal Ub. For mutational effects, red indicates drastic effects and pink indicates weak effects. Only one residue D32 with significant chemical shift perturbation is mapped to the reverse side of Ub (not shown).
Figure 7
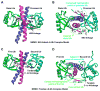
Model of NEMO: di-Ub interactions (A) The NEMO: K63-linked di-Ub interaction model produced by HADDOCK. Only the LZ region of NEMO dimer is shown in blue and magenta for each chain, respectively. The distal and the proximal Ub molecules are in green and cyan, respectively. Residues critical for NEMO interaction are labeled and shown in red. Those that are less critical for NEMO interaction are shown in pink, but not labeled. (B) Same as A, but rotated approximately 90° around the horizontal axis. The critical interaction patches are circled by ovals. (C) The NEMO: tandem di-Ub interaction model produced by HADDOCK. Only the LZ region of NEMO dimer is shown in blue and magenta for each chain, respectively. The first and the second Ub molecules are in green and cyan, respectively. Residues critical for NEMO interaction are labeled and shown in red. Those that are less critical for NEMO interaction are shown in pink, but not labeled. (D) Same as C, but rotated approximately 90° around the horizontal axis. The critical interaction patches are circled by ovals.
Similar articles
- Direct inhibition of NF-κB activation by peptide targeting the NOA ubiquitin binding domain of NEMO.
Chiaravalli J, Fontan E, Fsihi H, Coic YM, Baleux F, Véron M, Agou F. Chiaravalli J, et al. Biochem Pharmacol. 2011 Nov 1;82(9):1163-74. doi: 10.1016/j.bcp.2011.07.083. Epub 2011 Jul 23. Biochem Pharmacol. 2011. PMID: 21803029 - Mechanism underlying IκB kinase activation mediated by the linear ubiquitin chain assembly complex.
Fujita H, Rahighi S, Akita M, Kato R, Sasaki Y, Wakatsuki S, Iwai K. Fujita H, et al. Mol Cell Biol. 2014 Apr;34(7):1322-35. doi: 10.1128/MCB.01538-13. Epub 2014 Jan 27. Mol Cell Biol. 2014. PMID: 24469399 Free PMC article. - DARPin-assisted crystallography of the CC2-LZ domain of NEMO reveals a coupling between dimerization and ubiquitin binding.
Grubisha O, Kaminska M, Duquerroy S, Fontan E, Cordier F, Haouz A, Raynal B, Chiaravalli J, Delepierre M, Israël A, Véron M, Agou F. Grubisha O, et al. J Mol Biol. 2010 Jan 8;395(1):89-104. doi: 10.1016/j.jmb.2009.10.018. Epub 2009 Oct 23. J Mol Biol. 2010. PMID: 19854204 - Protein-protein interactions involving IKKgamma (NEMO) that promote the activation of NF-kappaB.
Shifera AS. Shifera AS. J Cell Physiol. 2010 Jun;223(3):558-61. doi: 10.1002/jcp.22105. J Cell Physiol. 2010. PMID: 20301198 Review. - Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity.
Karin M, Ben-Neriah Y. Karin M, et al. Annu Rev Immunol. 2000;18:621-63. doi: 10.1146/annurev.immunol.18.1.621. Annu Rev Immunol. 2000. PMID: 10837071 Review.
Cited by
- The Ubiquitin Tale: Current Strategies and Future Challenges.
Upadhyay A, Joshi V. Upadhyay A, et al. ACS Pharmacol Transl Sci. 2024 Sep 4;7(9):2573-2587. doi: 10.1021/acsptsci.4c00278. eCollection 2024 Sep 13. ACS Pharmacol Transl Sci. 2024. PMID: 39296276 Review. - Secondary interactions in ubiquitin-binding domains achieve linkage or substrate specificity.
Michel MA, Scutts S, Komander D. Michel MA, et al. Cell Rep. 2024 Aug 27;43(8):114545. doi: 10.1016/j.celrep.2024.114545. Epub 2024 Jul 23. Cell Rep. 2024. PMID: 39052481 Free PMC article. - NEMO reshapes the α-Synuclein aggregate interface and acts as an autophagy adapter by co-condensation with p62.
Furthmann N, Bader V, Angersbach L, Blusch A, Goel S, Sánchez-Vicente A, Krause LJ, Chaban SA, Grover P, Trinkaus VA, van Well EM, Jaugstetter M, Tschulik K, Damgaard RB, Saft C, Ellrichmann G, Gold R, Koch A, Englert B, Westenberger A, Klein C, Jungbluth L, Sachse C, Behrends C, Glatzel M, Hartl FU, Nakamura K, Christine CW, Huang EJ, Tatzelt J, Winklhofer KF. Furthmann N, et al. Nat Commun. 2023 Dec 19;14(1):8368. doi: 10.1038/s41467-023-44033-0. Nat Commun. 2023. PMID: 38114471 Free PMC article. - ALPK1 mutants causing ROSAH syndrome or Spiradenoma are activated by human nucleotide sugars.
Snelling T, Saalfrank A, Wood NT, Cohen P. Snelling T, et al. Proc Natl Acad Sci U S A. 2023 Dec 12;120(50):e2313148120. doi: 10.1073/pnas.2313148120. Epub 2023 Dec 7. Proc Natl Acad Sci U S A. 2023. PMID: 38060563 Free PMC article. - Mechanisms underlying linear ubiquitination and implications in tumorigenesis and drug discovery.
Li J, Liu S, Li S. Li J, et al. Cell Commun Signal. 2023 Nov 28;21(1):340. doi: 10.1186/s12964-023-01239-5. Cell Commun Signal. 2023. PMID: 38017534 Free PMC article. Review.
References
- Agou F, Ye F, Goffinont S, Courtois G, Yamaoka S, Israel A, Veron M. NEMO trimerizes through its coiled-coil C-terminal domain. J Biol Chem. 2002;277:17464–17475. - PubMed
- Cook WJ, Jeffrey LC, Kasperek E, Pickart CM. Structure of tetraubiquitin shows how multiubiquitin chains can be formed. J Mol Biol. 1994;236:601–609. - PubMed
- Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene. 2006;25:6831–6843. - PubMed
- Dominguez C, Boelens R, Bonvin AM. HADDOCK: a protein-protein docking approach based on biochemical or biophysical information. J Am Chem Soc. 2003;125:1731–1737. - PubMed
MeSH terms
Substances
Grants and funding
- R01 AG019391/AG/NIA NIH HHS/United States
- R37 AG019391/AG/NIA NIH HHS/United States
- R01 AG025440-04/AG/NIA NIH HHS/United States
- R01 AG019391-09/AG/NIA NIH HHS/United States
- R01 AG025440/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous