IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer - PubMed (original) (raw)
IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer
Sergei Grivennikov et al. Cancer Cell. 2009.
Erratum in
- Cancer Cell. 2009 Mar 3;15(3):241
Abstract
Colitis-associated cancer (CAC) is the most serious complication of inflammatory bowel disease. Proinflammatory cytokines have been suggested to regulate preneoplastic growth during CAC tumorigenesis. Interleukin 6 (IL-6) is a multifunctional NF-kappaB-regulated cytokine that acts on epithelial and immune cells. Using genetic tools, we now demonstrate that IL-6 is a critical tumor promoter during early CAC tumorigenesis. In addition to enhancing proliferation of tumor-initiating cells, IL-6 produced by lamina propria myeloid cells protects normal and premalignant intestinal epithelial cells (IECs) from apoptosis. The proliferative and survival effects of IL-6 are largely mediated by the transcription factor Stat3, whose IEC-specific ablation has profound impact on CAC tumorigenesis. Thus, the NF-kappaB-IL-6-Stat3 cascade is an important regulator of the proliferation and survival of tumor-initiating IECs.
Conflict of interest statement
All other authors declare no competing financial interests.
Figures
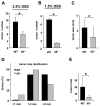
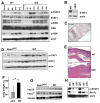
Figure 1. IL-6 controls tumor formation and growth in a mouse CAC model
WT and _Il6_-/- mice were subjected to AOM-based CAC induction protocol using three cycles of 2.5% (A) or 1.5% (B) DSS in drinking water. Tumors were counted at the end of the 12 week CAC induction regimen. Data represent average tumor numbers ± s.d., n>10. p=0.003 for (A); p=0.0001 for (B). (C) Tumor sizes were determined using imaging Spot software (Zeiss) for microscopic tumors or with a caliper for macroscopic tumors. Average tumor size ± s.d. is shown; * p=0.012. (D) Histogram showing size distribution of tumors. (E) Average tumor load was determined by summing all tumor diameters for a given animal. Results are averages ± s.d. (n>7); * p=0.047.
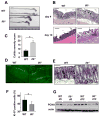
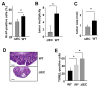
Figure 2. IL-6 is required for maintenance of mucosal integrity
(A) WT and _Il6_-/- mice exhibit colon shortening after 7 days of 2.5% DSS exposure. (B) Mucosal histology was examined in WT or _Il6_-/- mice 4 or 10 days after initiation of 2.5% DSS treatment by H&E staining of paraffine embedded sections. Scale bar- 50 μm. (C) Colitis severity score after 3% DSS exposure was determined on day 10. Results are averages ± s.d. (n=5), * p≤0.05. (D) Apoptosis in colons of 3% DSS treated mice was evaluated on day 4 after DSS administration by TUNEL staining. Scale bar- 50 μm. (E) The extent of IEC proliferation in colons of DSS-treated mice was determined by BrdU labeling and immunohistochemistry. Scale bar- 50 μm. (F) The percentage of Ki-67 positive cells among all crypt cells in colons of DSS treated mice was enumerated. Results are averages ± s.d. (n=6), * p= 0.001. (G) Lysates of distal colons prepared on the indicated days after initiation of 3% DSS treatment were analyzed for PCNA expression by immunoblotting.
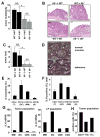
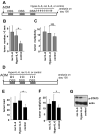
Figure 3. IL-6 produced by bone marrow derived cells is required for CAC tumorigenesis
(A) Tumor multiplicity in radiation chimeras subjected to induction of CAC using 2.5% DSS. Results are averages ± s.d. (n>8), * p<0.01. (B) Paraffine embedded sections of adenoma-containing colons stained with H&E. Scale bar- 100 μm. (C) Average tumor loads in radiation chimeras. Results are averages ± s.d. (n=5), NS- not significant, * p<0.01. (D) Immunohistochemical analysis of IL-6 expression in DSS-treated colons (normal mucosa) or CAC-bearing colons of WT mice. Scale bar- 50 μm. (E,F) Intracellular IL-6 cytokine staining of PMA+ionomycin restimulated tumor infiltrating cells analyzed by flow cytometry. Results are averages ± s.d. (n=3). (E) Percentages of IL-6 expressing cells in each given population (DC, macrophages, T and B cells) (F) Percentages of each population positive for IL-6 cells among all tumor infiltrating cells. (G) Macrophages (CD45+CD11b+CD11c+), dendritic cells (CD45+CD11b+CD11c+) and T cells (CD45+CD4+ or CD45+CD8α+) isolated by FACS sorting were analyzed for IL-6 mRNA by Q-RT-PCR. Tumor population- tumor infiltrating cells from pooled tumors. LP population- lamina propria cells from colons from which the tumors were excised. (H) Percentage of macrophages (CD11b+), T cells (CD3+) and dendritic cells (CD11c+) in total lamina propria cells isolated from pooled CAC tumors from WT mice.
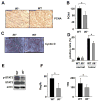
Figure 4. IL-6 regulates cell proliferation and expression of genes involved in proliferation, survival and inflammation
(A) Colons of adenoma-bearing mice were stained with anti-PCNA antibody. Scale bar- 50 μm. (B) Lysates of distal colons of adenoma-bearing mice were prepared and PCNA mRNA expression was analyzed by Q-RT-PCR. Results are averages ± s.d. (n=3). (C) Sections of adenoma-bearing colons were stained with antibodies to cyclin D. Scale bar- 50 μm. (D) Percentages of Ki-67-positive cells within colonic crypts. Results are averages ± s.d. (n=6), * p=0.005. (E) IEC from mice treated with 2.5% DSS were purified on day 10. Expression of the indicated proteins was analyzed by immunoblotting after gel separation. (F) RegIIIγ and Tff3 mRNA expression was analyzed by Q-RT-PCR. Results are averages ± s.d. (n=3).
Figure 5. STAT3 maintains mucosal integrity during acute colitis
(A). Mice were exposed to 2.5% DSS. Colonic lysates were prepared at the indicated timepoints and analyzed for expression and phosphorylation of the indicated proteins. (B) Deletion of STAT3 in IEC of _Stat3_ΔIEC mice was examined by immunoblotting of IEC lysates. (C) Deletion of STAT3 in colons of _Stat3_ΔIEC mice was analyzed by immunohistochemistry with anti-STAT3 antibody. Scale bar- 50 μm (D) Total colon lysates of WT and _Stat3_ΔIEC mice were analyzed for STAT3 expression and phosphorylation by immunoblot analysis (E) Increased susceptibility of _Stat3_ΔIEC mice to DSS colitis. Colons of WT and _Stat3_ΔIEC mice were analyzed by sectioning and H&E staining 10 days after initiation of 2.5% DSS exposure. Scale bar- 100 μm. (F) Bodyweights of the indicated mice were measured 10 days after initiation of 2.5% DSS exposure. Results show % of body weight on day 0 and are averages ± s.d. (n=6), p<0.05. (G;H) Total colon lysates (G) or IEC lysate (H) from mice on day 10 after 2.5% DSS administration were analyzed for expression of the indicated proteins by immunoblotting.
Figure 6. STAT3 is critical for CAC tumorigenesis
(A) Percentage of Ki-67-positive cells in WT and _Stat3_ΔIEC colonic crypts 10 days after initiation of DSS exposure. Results are averages ± s.d. (n=3). * p=0.03. (B) Tumor multiplicity in WT and _Stat3_ΔIEC mice subjected to induction of CAC. Results are averages ± s.d. (n=8), *p=0.004. (C) Tumor sizes in WT and _Stat3_ΔIEC mice. Results are averages ± s.d. (n=8), * p=0.012. (D) Paraffine embedded sections of adenoma-containing colons of WT and _Stat3_ΔIEC mice were stained with H&E. Scale bar- 100 μm. (E) Apoptosis in colons of DSS treated mice was evaluated on day 4 after DSS administration by TUNEL staining. Amount of TUNEL-positive cells per microscope field was determined. Results are averages ± s.d. (n=3). * p=0.05.
Figure 7. IL-6 signaling stimulates tumor formation and growth
(A) Scheme of treatment with IL-6 agonists during late stage of CAC growth. Mice were i.p. injected with 2 μg Hyper-IL-6 or 5 μg recombinant IL-6 every 3 days after the last DSS cycle. Tumors were analyzed on day 100 after AOM injection. (B) Numbers of tumors larger than > 2mm. Results are averages ± s.d. (n=7), * p<0.05. (C) Tumor multiplicity; Results are averages ± s.d., NS- not significant. (D) Scheme of treatment with IL-6 agonists during CAC induction. Mice were i.p. injected with the same amounts of IL-6 agonists as in (A), on day 1, 5 and 8 of each DSS cycle. Tumors were analyzed 100 days after AOM injection. (E) Average tumor load. Results are averages ± s.d. (n=6). * p<0.05. (F) Tumor multiplicity. Results are averages ± s.d. (n=6). * p<0.05. (G) Immunoblot analysis of colonic lysates from mice treated with hyper-IL-6, rec IL-6 or PBS after exposure to 2.5% DSS for 7 days. Mice were sacrificed on day 10 30 min after the last treatment.
Comment in
- Inflammation and cancer: IL-6 and STAT3 complete the link.
Bromberg J, Wang TC. Bromberg J, et al. Cancer Cell. 2009 Feb 3;15(2):79-80. doi: 10.1016/j.ccr.2009.01.009. Cancer Cell. 2009. PMID: 19185839 Free PMC article.
References
- Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, Schutz M, Bartsch B, Holtmann M, Becker C, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. - PubMed
- Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187–196. - PubMed
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. - PubMed
- Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous