Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment - PubMed (original) (raw)
Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment
Marcin Kortylewski et al. Cancer Cell. 2009.
Erratum in
- Cancer Cell. 2010 Nov 16;18(5):536
Abstract
Interactions between tumor and immune cells either enhance or inhibit cancer progression. We show here that Stat3 signaling within the tumor microenvironment induces a procarcinogenic cytokine, IL-23, while inhibiting a central anticarcinogenic cytokine, IL-12, thereby shifting the balance of tumor immunity toward carcinogenesis. Stat3 induces expression of IL-23, which is mainly produced by tumor-associated macrophages, via direct transcriptional activation of the IL-23/p19 gene. Furthermore, Stat3 inhibits NF-kappaB/c-Rel-dependent IL-12/p35 gene expression in tumor-associated dendritic cells. Tumor-associated regulatory T cells (Tregs) express IL-23 receptor, which activates Stat3 in this cell type, leading to upregulation of the Treg-specific transcription factor Foxp3 and the immunosuppressive cytokine IL-10. These results demonstrate that Stat3 promotes IL-23-mediated procarcinogenic immune responses while inhibiting IL-12-dependent antitumor immunity.
Figures
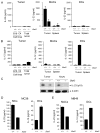
Figure 1. IL-23 and IL-12 expression in tumor-infiltrating myeloid cells is compartmentalized and oppositely regulated by Stat3
ELISA measurement of protein levels of IL-23 (A) and IL-12 (B) in supernatants of tumor cell lines (B16 and C4 melanoma cells), B16 whole tumor cell suspensions (Total tumor) from mice with Stat3+/+ or _Stat3_−/− hematopoietic cells, enriched tumor-infiltrating CD11b+CD11c− macrophages (MACs) and CD11c+ dendritic cells (DCs) cultured in vitro for 24 h. Data shown are mean values ± SD from one of three separate experiments performed on cells pooled from four animals per group analyzed. (C) Western blot detection of IL-23/p19 protein levels in the CD11b+ cells isolated from the whole B16 tumor and tumor-draining lymph nodes of Stat3+/+ or _Stat3_−/− mice. (D, E) IL-23 and IL-12 protein levels measured by ELISA as in Fig. 1A, in supernatants from the enriched tumor-infiltrating CD11b+CD11c− MACs and CD11c+ DCs isolated from the whole MC38 colon carcinoma (D) and MB49 bladder carcinoma (E) tumors; mean ± SEM (n = 3).
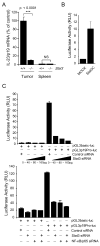
Figure 2. Stat3 and NF-κB/p65 synergistically enhance IL-23 expression by directly binding to the IL-23/p19 promoter
(A) Stat3 upregulates expression of IL-23/p19 mRNA in CD11b+ myeloid cells freshly isolated from B16 tumors. Shown are the results from one of three independent experiments analyzed by real-time PCR, normalized to 18S rRNA. (B) Over expression of constitutively active Stat3 mutant (Stat3C) activates transcription by IL-23/p19 promoter. The fragment from the mouse promoter of IL-23/p19 (sequence from -1159 to +160) was cloned into pGL3 vector with luciferase reporter gene. Dual-luciferase activity was determined 24 h after transfection of various sets of expression vectors into 3T3 fibroblasts. (C) Stat3 and NF-κB/p65 bind directly to the p19 promoter. Top: Stat3 silencing downregulates the activity of IL-23/p19 promoter. Dual-luciferase activity was measured in lysates of B16 cells 24 h after transfection with various concentrations of Stat3 siRNA or with scrambled RNA control. Bottom: Both Stat3 and NF-κB/p65 transcription factors are required for the transcriptional activity of IL-23/p19 promoter. Stat3 siRNA, NF-κB/p65 siRNA or both were transfected into B16 cells together with IL-23/p19 promoter-luciferase construct. Dual-luciferase activity was measured as described above. Data shown are mean values ± SD from experiments performed in triplicates.
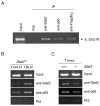
Figure 3. Both Stat3 and NF-κB/p65 bind to the IL-23/p19 promoter
(A) B16 tumor cells were transfected with pRV-CMV Stat3-Flag or control vector, and antibodies specific for Stat3, Flag-M2, NF-κB/p65 or control IgG as indicated, were used for chromatin immunoprecipitation (ChIP). Chromatin was purified and amplified by PCR using primers specific for Stat3 and NF-κB binding sequence of the mouse IL-23/p19 promoter. (B) The binding of Stat3 and NF-κB/p65 to IL-23/p19 promoter is elevated in tumor-draining but not in distant lymph nodes. Cells isolated from pooled tumor draining or contralateral lymph nodes were fixed with formaldehyde and subjected to ChIP assay performed as described above. (C) Tumors from mice with myeloid cell-specific Stat3 ablation show reduced Stat3 and NF-κB/p65 binding to the IL-23/p19 promoter as assessed by ChIP assay.
Figure 4. Stat3 inhibits IL-12/p35 gene expression
(A) Stat3 knockdown or ablation augments IL-12/p35 expression in the whole tumor (left panel) and in the tumor infiltrating myeloid cells (right panel); mean ± SD (n = 3). (B) Stat3 inhibits c-Rel activity and binding of c-Rel to IL-12/p35 promoter in tumors. Left panel: Western blot analysis of c-Rel phosphorylation after immunoprecipitation of total c-Rel from spleens and tumors grown in mice with Stat3+/+ and _Stat3_−/− hematopoietic compartment. Right panel: Ablating Stat3 (in hematopoietic cells) increases binding of NF-κB/c-Rel, but not NF-κB/p65, to IL-12/p35 promoter in whole tumor preparation, as measured by ChIP assay.
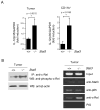
Figure 5. IL-23 receptor and Stat3 signaling in tumor Tregs
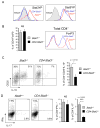
(A) Stat3 is activated in tumor Tregs, which are IL-23R positive. Cell suspensions prepared from spleens and from tumors as well as tumor draining lymph nodes (TDLN) were subjected to flow analyses for phospho-Stat3 (top panel) and IL-23R (three bottom panels). (B) Recombinant IL-23 can further activate Stat3 in CD4+Foxp3+ lymphocytes derived from tumors but not spleens. Shown are the representative results from 2–3 independent experiments.
Figure 6. The effects of Stat3 ablation on tumor-infiltrating Tregs
(A) Stat3 ablation does not significantly affect Stat5 activity in tumor-infiltrating CD4+Foxp3+ Tregs as measured by intracellular staining with Stat3- and Stat5-phosphospecific antibody staining and flow cytometry. (B) The effect of Stat3 signaling in tumor CD4+ T cells on the number of FoxP3+ cells (left) and expression levels of FoxP3 (right), using CD4+ T cells prepared from B16 tumors grown in Stat3flox/flox (Stat3+/+) and CD4cre/Stat3flox/flox mice. Right panel: representative histogram for one of 3 independent experiments with total n = 12; mean ± SEM; NS, not significant. (C) Lower expression of IL-10 by tumor-derived CD4+CD25+ Tregs from _Stat3_−/− mice. Left two panels – shown are representative results for 3 independent experiments. Right bar graph: combining 3 independent experiments with total n = 12; mean ± SEM; p = 0.0103. (D) Intracellular expression of IFNγ and IL-17 cytokines by tumor-infiltrating Stat3-positive and Stat3-negative CD4+ T cells, as assessed by intracellular staining and flow cytometry using freshly isolated whole tumor cell preparations (left two panels). Right two bar graphs represent 3 independent experiments, with total n = 12, p > 0.05 and p < 0.0001, respectively; NS, not significant. Shown are mean values ± SEM
Figure 7. Blocking IL-23R signaling in vivo affects tumor Tregs and tumor growth
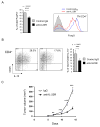
Mice were challenged with B16 tumors injected s.c. and treated with IL-23R-specific neutralizing antibodies or with rat IgG2b control antibodies. (A) Blocking the IL-23 signaling reduces numbers of tumor-infiltrating Tregs (left panel) and, especially expression level of FoxP3 (right panel). (B) IL-23R neutralization reduces IL-10 production by CD4+CD25+ Tregs. The phenotypic analysis of tumor-infiltrating CD4+ T cells were accomplished by cell surface/intracellular staining with specific antibodies and flow cytometry. For both (A) and (B), shown are representative results of FACS analysis from one of two independent experiments. Bar graphs show the means ± SEM with total n = 7, p < 0.0001 (A) and p = 0.0003 (B). (C) Effects of IL-23R blockade on B16 tumor growth. Shown are combined results of two independent experiments as means ± SEM; total n = 10; ***, p < 0.001 and **, p < 0.01.
Comment in
- Reinforcing suppression using regulators: a new link between STAT3, IL-23, and Tregs in tumor immunosuppression.
Stewart CA, Trinchieri G. Stewart CA, et al. Cancer Cell. 2009 Feb 3;15(2):81-3. doi: 10.1016/j.ccr.2009.01.008. Cancer Cell. 2009. PMID: 19185840
References
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. - PubMed
- Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. - PubMed
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. - PubMed
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous