CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance - PubMed (original) (raw)
Comparative Study
. 2009 Feb 16;206(2):421-34.
doi: 10.1084/jem.20081811. Epub 2009 Feb 2.
Affiliations
- PMID: 19188497
- PMCID: PMC2646578
- DOI: 10.1084/jem.20081811
Comparative Study
CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance
Randall H Friedline et al. J Exp Med. 2009.
Erratum in
- J Exp Med. 2009 Mar 16;206(3):721
Abstract
Cytotoxic T lymphocyte antigen-4 (CTLA-4) plays a critical role in negatively regulating T cell responses and has also been implicated in the development and function of natural FOXP3(+) regulatory T cells. CTLA-4-deficient mice develop fatal, early onset lymphoproliferative disease. However, chimeric mice containing both CTLA-4-deficient and -sufficient bone marrow (BM)-derived cells do not develop disease, indicating that CTLA-4 can act in trans to maintain T cell self-tolerance. Using genetically mixed blastocyst and BM chimaeras as well as in vivo T cell transfer systems, we demonstrate that in vivo regulation of Ctla4(-/-) T cells in trans by CTLA-4-sufficient T cells is a reversible process that requires the persistent presence of FOXP3(+) regulatory T cells with a diverse TCR repertoire. Based on gene expression studies, the regulatory T cells do not appear to act directly on T cells, suggesting they may instead modulate the stimulatory activities of antigen-presenting cells. These results demonstrate that CTLA-4 is absolutely required for FOXP3(+) regulatory T cell function in vivo.
Figures
Figure 1.
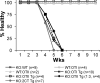
Ctla4+/+ cells can regulate Ctla4−/− T cells in mixed BM and blastocyst chimaeras. (A) Healthy Rag1−/− mice received a total of ∼4 × 106 T cell–depleted BM cells from 2–3-wk-old Ctla4−/− mice (KO only) or a 1:1 mix from Ctla4−/− and WT mice (KO:WT). Blastocyst chimaeras were generated as per Materials and methods, and all chimaeras were monitored for disease incidence. Mice were considered healthy (% Healthy) if no evidence of prolonged weight loss (>2 wk), ruffled fur, hunched posture, or skin or eye inflammation were visible. Mice were also bled periodically to measure relative KO:WT T cell ratios. _Ctla4−/−_-only recipients were killed for ethical reasons by 8 wk of age. (B) 12 blastocyst chimaeras, each line representing individual mice, were periodically bled over several years, and the frequency of Ctla4−/− T cells among total T cells enumerated via flow cytometry. (C) 6–8-wk-old KO only or KO:WT chimaera groups were killed, and LN cells were isolated and analyzed for expression of activation markers CD44, CD62L, CD69, and incorporation of the thymidine analogue BrdU, by flow cytometry. Results gated on CD4+ LN T cells from representative chimaeras are shown. Data shown in A and C are representative of over a dozen experiments. Data in B are the cumulative results of at least three experiments.
Figure 2.
A diverse repertoire of Ctla4+/+ T cells are required to regulate Ctla4−/− T cells in vivo. Rag1−/− recipient mice received a total of ∼4 × 106 T cell–depleted BM cells from a 1:1 mix of Ctla4−/− and WT mice (KO:WT), or Ctla4−/− and 2CT, OTI, or OTII TCR transgenic Rag1/2-sufficient mice. Some mice received a 1:3 ratio of Ctla4−/−:OTII BM cells. Mice were monitored for disease incidence (% Healthy), as described in Fig. 1 A. Data are pooled from two independent experiments.
Figure 3.
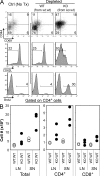
Ctla4−/− T cell quiescence depends on the persistent presence of WT T cells. (A) WT_:_WT or Ctla4−/−:WT (KO:WT) mixed BM chimaeras received depleting anti-CD90.1 mAb (no treatment control, PBS injected only, and depleted) as indicated in Materials and methods. 8 wk after initiating depletion, chimaeras were killed and analyzed. LN cells were isolated and stained for CD44, CD62L, and CD69. Incorporation of the thymidine analogue BrdU as a measure of cell turnover was also assayed. Data are gated on CD90.2+ CD4+ T cells. (B) Enumeration of T cells from the LN and spleens (SN) of depleted mice. Each circle represents individual mice. Data are representative of two independent experiments with at least three chimaeras per group.
Figure 4.
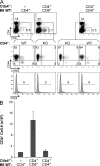
WT CD4+ T cells regulate Ctla4−/− T cells in vivo. CD4+ or CD8+ T cells from WT mice and CD4+ Ctla4−/− T cells from mixed BM chimaeras were sorted and transferred (107 cells total) to Rag1−/− recipients. (A) Representative cytometric data showing the activation profile of CD4+ T cells and the frequencies of CD25+ CD4+ T cells from each of the three recipient groups (WT CD4+ T cells alone, Ctla4−/− CD4+ and WT CD8+, and Ctla4−/− CD4+ and WT CD4+) 3 wk after transfer. (B) Splenic CD4+ T cell numbers from each group in A. Each group consisted of a minimum of two mice each and is representative of three experiments. Error bars denote the SD.
Figure 5.
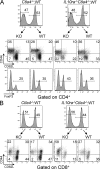
CD4+25+ WT T cells are able to regulate Ctla4−/− T cells in vivo. (A) WT T cell–depleted LN and spleen cells (KO) from healthy _Ctla4−/−:_WT mixed BM chimaeras were transferred (2 × 107 cells/mouse) either separately or with purified WT CD4+25+ T cells (2 × 106 cells/mouse) into Rag1−/− recipients. Frequency and activation status of CD4+ (A) and CD8+ (C) T cells from the LNs were analyzed in recipients 12 wk after transfer. (B) Quantitation of total, CD4+, and CD8+ T cell numbers in the spleen and LN of recipients at the time of analysis. Data are representative of three individual experiments with at least three mice per group. Error bars denote the SD.
Figure 6.
Expression of Gadd45β is decreased in naive Ctla4−/− T cells from mixed BM chimaeras relative to the WT T cells. Semiquantitative RT-PCR analysis of the expression of select genes in regulated Ctla4−/− T cells from mixed BM chimaeras with inconsistent results from the array experiments. Threefold dilutions of cDNA prepared from naive (CD44−CD62L+) WT or Ctla4−/− T cells were analyzed for the genes indicated with documented functions in T cell activation and tolerance. Expression of β-actin was used as loading control. Data are representative of three independent experiments.
Figure 7.
Regulation of Ctla4−/− T cells does not absolutely require signaling through the IL-10 receptor. Rag1−/− mice received a 1:1 mix of 4 × 106 BM cells from WT and either Ctla4−/− or Il10ra−/−Ctla4−/− mice. Mice were killed at 12 wk after reconstitution, and CD4+FoxP3− (A) or CD8+ (B) splenic T cells were analyzed for the activation markers CD44 and CD62L in WT and KO T cell populations. FoxP3+ frequency was also assessed in the CD4+ T cell subset (A, bottom). Data shown are representative flow cytometric results from the spleen of reconstituted mice (three mice per group) in two independent experiments.
Similar articles
- CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms.
Ise W, Kohyama M, Nutsch KM, Lee HM, Suri A, Unanue ER, Murphy TL, Murphy KM. Ise W, et al. Nat Immunol. 2010 Feb;11(2):129-35. doi: 10.1038/ni.1835. Epub 2009 Dec 27. Nat Immunol. 2010. PMID: 20037585 Free PMC article. - Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity.
Jain N, Nguyen H, Chambers C, Kang J. Jain N, et al. Proc Natl Acad Sci U S A. 2010 Jan 26;107(4):1524-8. doi: 10.1073/pnas.0910341107. Epub 2010 Jan 4. Proc Natl Acad Sci U S A. 2010. PMID: 20080649 Free PMC article. - Expression of CTLA-4 and FOXP3 in cis protects from lethal lymphoproliferative disease.
Chikuma S, Bluestone JA. Chikuma S, et al. Eur J Immunol. 2007 May;37(5):1285-9. doi: 10.1002/eji.200737159. Eur J Immunol. 2007. PMID: 17429849 - Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation.
Wing K, Yamaguchi T, Sakaguchi S. Wing K, et al. Trends Immunol. 2011 Sep;32(9):428-33. doi: 10.1016/j.it.2011.06.002. Epub 2011 Jun 30. Trends Immunol. 2011. PMID: 21723783 Review. - A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3.
Fontenot JD, Rudensky AY. Fontenot JD, et al. Nat Immunol. 2005 Apr;6(4):331-7. doi: 10.1038/ni1179. Nat Immunol. 2005. PMID: 15785758 Review.
Cited by
- Adoptive Transfer of Regulatory Immune Cells in Organ Transplantation.
Oberholtzer N, Atkinson C, Nadig SN. Oberholtzer N, et al. Front Immunol. 2021 Mar 2;12:631365. doi: 10.3389/fimmu.2021.631365. eCollection 2021. Front Immunol. 2021. PMID: 33737934 Free PMC article. Review. - Gene expression profile of peripheral blood lymphocytes from renal cell carcinoma patients treated with IL-2, interferon-α and dendritic cell vaccine.
Wolf B, Schwarzer A, Côté AL, Hampton TH, Schwaab T, Huarte E, Tomlinson CR, Gui J, Fisher JL, Fadul CE, Hamilton JW, Ernstoff MS. Wolf B, et al. PLoS One. 2012;7(12):e50221. doi: 10.1371/journal.pone.0050221. Epub 2012 Dec 3. PLoS One. 2012. PMID: 23226513 Free PMC article. - The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network.
Voskens CJ, Goldinger SM, Loquai C, Robert C, Kaehler KC, Berking C, Bergmann T, Bockmeyer CL, Eigentler T, Fluck M, Garbe C, Gutzmer R, Grabbe S, Hauschild A, Hein R, Hundorfean G, Justich A, Keller U, Klein C, Mateus C, Mohr P, Paetzold S, Satzger I, Schadendorf D, Schlaeppi M, Schuler G, Schuler-Thurner B, Trefzer U, Ulrich J, Vaubel J, von Moos R, Weder P, Wilhelm T, Göppner D, Dummer R, Heinzerling LM. Voskens CJ, et al. PLoS One. 2013;8(1):e53745. doi: 10.1371/journal.pone.0053745. Epub 2013 Jan 14. PLoS One. 2013. PMID: 23341990 Free PMC article. - CD28 co-stimulation in T-cell homeostasis: a recent perspective.
Beyersdorf N, Kerkau T, Hünig T. Beyersdorf N, et al. Immunotargets Ther. 2015 May 28;4:111-22. doi: 10.2147/ITT.S61647. eCollection 2015. Immunotargets Ther. 2015. PMID: 27471717 Free PMC article. Review. - Modulation of Phenotype and Function of Human CD4+CD25+ T Regulatory Lymphocytes Mediated by cAMP-Elevating Agents.
Riccomi A, Gesa V, Sacchi A, De Magistris MT, Vendetti S. Riccomi A, et al. Front Immunol. 2016 Sep 20;7:358. doi: 10.3389/fimmu.2016.00358. eCollection 2016. Front Immunol. 2016. PMID: 27703455 Free PMC article.
References
- Starr T.K., Jameson S.C., Hogquist K.A. 2003. Positive and negative selection of T cells.Annu. Rev. Immunol. 21:139–176 - PubMed
- Takeda S., Rodewald H., Arakawa H., Bluethmann H., Shimizu T. 1996. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span.Immunity. 5:217–228 - PubMed
- Rooke R., Waltzinger C., Benoist C., Mathis D. 1997. Targeted complementation of MHC class II deficiency by intrathymic delivery of recombinant adenoviruses.Immunity. 7:123–134 - PubMed
- Tanchot C., Lemonnier F.A., Perarnau B., Freitas A.A., Rocha B. 1997. Differential requirements for survival and proliferation of CD8 naive or memory T cells.Science. 276:2057–2062 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials