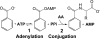Arabidopsis GH3.12 (PBS3) conjugates amino acids to 4-substituted benzoates and is inhibited by salicylate - PubMed (original) (raw)
Arabidopsis GH3.12 (PBS3) conjugates amino acids to 4-substituted benzoates and is inhibited by salicylate
Rachel A Okrent et al. J Biol Chem. 2009.
Abstract
Salicylate (SA, 2-hydroxybenzoate) is a phytohormone best known for its role as a critical mediator of local and systemic plant defense responses. In response to pathogens such as Pseudomonas syringae, SA is synthesized and activates widespread gene expression. In gh3.12/pbs3 mutants of Arabidopsis thaliana, induced total SA accumulation is significantly compromised as is SA-dependent gene expression and plant defense. AtGH3 subfamily I and II members have been shown to conjugate phytohormone acyl substrates to amino acids in vitro, with this role supported by in planta analyses. Here we sought to determine the in vitro biochemical activity and kinetic properties of GH3.12/avrPphB susceptible 3 (PBS3), a member of the uncharacterized AtGH3 subfamily III. Using a novel high throughput adenylation assay, we characterized the acyl substrate preference of PBS3. We found PBS3 favors 4-substituted benzoates such as 4-aminobenzoate and 4-hydroxybenzoate, with moderate activity on benzoate and no observed activity with 2-substituted benzoates. Similar to known GH3 enzymes, PBS3 catalyzes the conjugation of specific amino acids (e.g. Glu) to its preferred acyl substrates. Kinetic analyses indicate 4-aminobenzoate and 4-hydroxybenzoate are preferred acyl substrates as PBS3 exhibits both higher affinities (apparent K(m) = 153 and 459 microm, respectively) and higher catalytic efficiencies (k(cat)/K(m) = 0.0179 and 0.0444 microm(-1) min(-1), respectively) with these acyl substrates compared with benzoate (apparent K(m) = 867 microm, k(cat)/K(m) = 0.0046 microm(-1) min(-1)). Notably, SA specifically and reversibly inhibits PBS3 activity with an IC(50) of 15 microm. This suggests a general mechanism for the rapid, reversible regulation of GH3 activity and small molecule cross-talk. For PBS3, this may allow for coordination of flux through diverse chorismate-derived pathways.
Figures
FIGURE 1.
Reaction catalyzed by GH3 enzymes, shown with benzoate as the acyl substrate. Adenylation of the acyl substrate is followed by formation of the amino acid conjugate.
FIGURE 2.
PBS3 catalyzes the formation of amino acid conjugates with preferred acyl substrates. A, TLC analysis of 4-HBA amino acid conjugate formation by PBS3. B, HPLC analysis of 4-HBA-Glu formation by PBS3.Inset, verification of putative 4-HBA-Glu peak by LC-MS. Shown is Q-TOF MS/MS on m/z = 268.1 with 4-HBA-Glu structure. Likely fission site is indicated. C, TLC analysis of pABA-Glu formation by PBS3 as follows: pABA standard (left), reaction mixture (middle), and pABA-Glu standard (right). D, TLC analysis of the PBS3 reactions with vanillic acid (VA), 4-HBA, or_trans-_cinnamic acid (t-CA) as the acyl substrate and Glu. The arrow indicates a faint spot corresponding to_trans-_cinnamic acid-Glu. E, TLC of the PBS3 reaction with 4-HBA and Glu derivatives. AAD,
l
-2-aminoadipic acid;Gla, γ-carboxyl
l
-glutamic acid; MeGlu,
l
-gutamic acid γ-methyl ester. Images of TLC plates were converted to grayscale and adjustments of “levels” in Photoshop were performed. Independent experiments with different batches of purified, recombinant PBS3 produced similar results.
FIGURE 3.
Inhibition of PBS3 activity by SA. SA inhibits the formation of pABA-Glu catalyzed by PBS3. The reaction velocities were determined by monitoring the formation of pABA-Glu by HPLC of PBS3 reactions with 150 μ
m
pABA and 10 m
m
Glu. Independent experiments yielded similar results.
Similar articles
- Preference of Arabidopsis thaliana GH3.5 acyl amido synthetase for growth versus defense hormone acyl substrates is dictated by concentration of amino acid substrate aspartate.
Mackelprang R, Okrent RA, Wildermuth MC. Mackelprang R, et al. Phytochemistry. 2017 Nov;143:19-28. doi: 10.1016/j.phytochem.2017.07.001. Epub 2017 Jul 23. Phytochemistry. 2017. PMID: 28743075 - The GH3 acyl adenylase family member PBS3 regulates salicylic acid-dependent defense responses in Arabidopsis.
Nobuta K, Okrent RA, Stoutemyer M, Rodibaugh N, Kempema L, Wildermuth MC, Innes RW. Nobuta K, et al. Plant Physiol. 2007 Jun;144(2):1144-56. doi: 10.1104/pp.107.097691. Epub 2007 Apr 27. Plant Physiol. 2007. PMID: 17468220 Free PMC article. - Brassicaceae-specific Gretchen Hagen 3 acyl acid amido synthetases conjugate amino acids to chorismate, a precursor of aromatic amino acids and salicylic acid.
Holland CK, Westfall CS, Schaffer JE, De Santiago A, Zubieta C, Alvarez S, Jez JM. Holland CK, et al. J Biol Chem. 2019 Nov 8;294(45):16855-16864. doi: 10.1074/jbc.RA119.009949. Epub 2019 Oct 1. J Biol Chem. 2019. PMID: 31575658 Free PMC article. - Biosynthesis of salicylic acid in plants.
Chen Z, Zheng Z, Huang J, Lai Z, Fan B. Chen Z, et al. Plant Signal Behav. 2009 Jun;4(6):493-6. doi: 10.4161/psb.4.6.8392. Epub 2009 Jun 12. Plant Signal Behav. 2009. PMID: 19816125 Free PMC article. Review. - The GH3 amidosynthetases family and their role in metabolic crosstalk modulation of plant signaling compounds.
Wojtaczka P, Ciarkowska A, Starzynska E, Ostrowski M. Wojtaczka P, et al. Phytochemistry. 2022 Feb;194:113039. doi: 10.1016/j.phytochem.2021.113039. Epub 2021 Dec 1. Phytochemistry. 2022. PMID: 34861536 Review.
Cited by
- Glutamate receptor-like channel3.3 is involved in mediating glutathione-triggered cytosolic calcium transients, transcriptional changes, and innate immunity responses in Arabidopsis.
Li F, Wang J, Ma C, Zhao Y, Wang Y, Hasi A, Qi Z. Li F, et al. Plant Physiol. 2013 Jul;162(3):1497-509. doi: 10.1104/pp.113.217208. Epub 2013 May 8. Plant Physiol. 2013. PMID: 23656893 Free PMC article. - Salicylic Acid Binding Proteins (SABPs): The Hidden Forefront of Salicylic Acid Signalling.
Pokotylo I, Kravets V, Ruelland E. Pokotylo I, et al. Int J Mol Sci. 2019 Sep 6;20(18):4377. doi: 10.3390/ijms20184377. Int J Mol Sci. 2019. PMID: 31489905 Free PMC article. Review. - Arabidopsis auxin mutants are compromised in systemic acquired resistance and exhibit aberrant accumulation of various indolic compounds.
Truman WM, Bennett MH, Turnbull CG, Grant MR. Truman WM, et al. Plant Physiol. 2010 Mar;152(3):1562-73. doi: 10.1104/pp.109.152173. Epub 2010 Jan 15. Plant Physiol. 2010. PMID: 20081042 Free PMC article. - Auxins and Cytokinins-The Role of Subcellular Organization on Homeostasis.
Skalický V, Kubeš M, Napier R, Novák O. Skalický V, et al. Int J Mol Sci. 2018 Oct 11;19(10):3115. doi: 10.3390/ijms19103115. Int J Mol Sci. 2018. PMID: 30314316 Free PMC article. Review. - Multiple roles of WIN3 in regulating disease resistance, cell death, and flowering time in Arabidopsis.
Wang GF, Seabolt S, Hamdoun S, Ng G, Park J, Lu H. Wang GF, et al. Plant Physiol. 2011 Jul;156(3):1508-19. doi: 10.1104/pp.111.176776. Epub 2011 May 4. Plant Physiol. 2011. PMID: 21543726 Free PMC article.
References
- Glazebrook, J. (2001) Curr. Opin. Plant Biol. 4 301–308 - PubMed
- Hagen, G., Kleinschmidt, A., and Guilfoyle, T. (1984) Planta 162 147–153 - PubMed
- Terol, J., Domingo, C., and Talon, M. (2006) Gene (Amst.) 371 279–290 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous