Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats - PubMed (original) (raw)
Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats
Gabriel Paulino et al. Am J Physiol Endocrinol Metab. 2009 Apr.
Abstract
The vagal afferent pathway is important in short-term regulation of food intake, and decreased activation of this neural pathway with long-term ingestion of a high-fat diet may contribute to hyperphagic weight gain. We tested the hypothesis that expression of genes encoding receptors for orexigenic factors in vagal afferent neurons are increased by long-term ingestion of a high-fat diet, thus supporting orexigenic signals from the gut. Obesity-prone (DIO-P) rats fed a high-fat diet showed increased body weight and hyperleptinemia compared with low-fat diet-fed controls and high-fat diet-induced obesity-resistant (DIO-R) rats. Expression of the type I cannabinoid receptor and growth hormone secretagogue receptor 1a in the nodose ganglia was increased in DIO-P compared with low-fat diet-fed controls or DIO-R rats. Shifts in the balance between orexigenic and anorexigenic signals within the vagal afferent pathway may influence food intake and body weight gain induced by high fat diets.
Figures
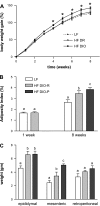
Fig. 1.
Effect of ingestion of a high-fat (HF) diet on body weight gain, adiposity, and fat pad mass in low-fat (LF) and HF rats after 1 and 8 wk on respective diets. A: diet-induced obesity-prone (DIO-P) rats had a significant increase in body weight (expressed as a percentage of initial body weight) at 3–8 wk compared with diet-induced obesity-resistant (DIO-R) or LF rats (LF or DIO-R vs. DIO-P, P < 0.05). B: adiposity index calculated as the sum of fat pads expressed as a percentage of body weight. Note that at week 1, rats divided into 2 groups, either LF or HF, as cannot be discriminated into DIO-R and DIO-P groups. There was a significant increase in adiposity in DIO-R and DIO-P rats compared with rats maintained on LF diet (LF vs. DIO-R or DIO-P, P < 0.05). C: mass of different fat pads. There was a significant difference in mesenteric fat pad mass between DIO-R and DIO-P rats after 8 wk on a HF diet (P < 0.05). Data are means ± SE (LF, n = 10; DIO-R, n = 7; and DIO-P, n = 8). a,b,cDifferent letters denote significant differences between groups.
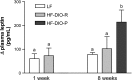
Fig. 2.
The increase in plasma leptin concentration between fasted state and 2 h after oral lipid gavage was significantly higher in DIO-P than in LF or DIO-R rats after 8 wk on a HF diet. Data are means ± SE (week 1: LF, n = 5; HF, n = 5; week 8: LF, n = 4; DIO-R and DIO-P, n = 5). a,b_P_ < 0.05, different letters denote significant differences between groups.
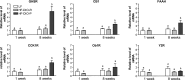
Fig. 3.
Cholecystokinin receptor (CCK1R), growth hormone secretagogue receptor (GHSR), cannabinoid type 1 receptor (CB1), and fatty acid amide hydrolase (FAAH) expression in the nodose ganglia are significantly increased in DIO-P but not DIO-R rats. No change was observed for Y2 receptor and leptin receptor (Ob1R). Receptor expression was expressed relative to LF rats at week 1 as a control group. Data are means ± SE (week 1: LF, n = 5; HF, n = 5; week 8: LF, n = 4; DIO-R, n = 5; DIO-P, n = 5).
Fig. 4.
Principle component analysis of all measured parameters from all rat groups after vast scale transformation of the data reveals the phenotypic shifts responsible for group changes in a single evaluation. Within-group variance was equivalent after transformation, and experimental groups are clearly discriminated. The first 2 principle components accounted for 86% of the variance in the data set. The 8-wk HF DIO-R animals differed from the 8-wk LF group in PC2 (P = 0.03) but not PC1 (P = 0.6), whereas the 8-wk HF DIO-P group differed from the LF group in both components (P < 0.001).
Fig. 5.
Correlations between variables were assessed in a Pearson's correlation matrix with all measured variables. Notably, fasting leptin was strongly correlated with epididymal fat mass but weakly correlated with other adipose depots measured. Also of specific interest, the change in CCK1R expression in nodose ganglion was strongly correlated with the fasting to 2-h post-lipid challenge leptin.
Similar articles
- High fat diet and body weight have different effects on cannabinoid CB(1) receptor expression in rat nodose ganglia.
Cluny NL, Baraboi ED, Mackie K, Burdyga G, Richard D, Dockray GJ, Sharkey KA. Cluny NL, et al. Auton Neurosci. 2013 Dec;179(1-2):122-30. doi: 10.1016/j.autneu.2013.09.015. Epub 2013 Oct 1. Auton Neurosci. 2013. PMID: 24145047 Free PMC article. - Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons.
de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. de Lartigue G, et al. Am J Physiol Endocrinol Metab. 2011 Jul;301(1):E187-95. doi: 10.1152/ajpendo.00056.2011. Epub 2011 Apr 26. Am J Physiol Endocrinol Metab. 2011. PMID: 21521717 Free PMC article. - High fat diet induced obesity alters endocannabinoid and ghrelin mediated regulation of components of the endocannabinoid system in nodose ganglia.
Christie S, O'Rielly R, Li H, Wittert GA, Page AJ. Christie S, et al. Peptides. 2020 Sep;131:170371. doi: 10.1016/j.peptides.2020.170371. Epub 2020 Jul 10. Peptides. 2020. PMID: 32659299 - Inducible nitric oxide synthase-derived nitric oxide reduces vagal satiety signalling in obese mice.
Yu Y, Park SJ, Beyak MJ. Yu Y, et al. J Physiol. 2019 Mar;597(6):1487-1502. doi: 10.1113/JP276894. Epub 2018 Dec 18. J Physiol. 2019. PMID: 30565225 Free PMC article. - Brain-gut axis and its role in the control of food intake.
Konturek SJ, Konturek JW, Pawlik T, Brzozowski T. Konturek SJ, et al. J Physiol Pharmacol. 2004 Mar;55(1 Pt 2):137-54. J Physiol Pharmacol. 2004. PMID: 15082874 Review.
Cited by
- Physiopathological Roles of White Adiposity and Gut Functions in Neuroinflammation.
Spinedi E, Docena GH. Spinedi E, et al. Int J Mol Sci. 2024 Oct 31;25(21):11741. doi: 10.3390/ijms252111741. Int J Mol Sci. 2024. PMID: 39519291 Free PMC article. Review. - Position statement on nutrition therapy for overweight and obesity: nutrition department of the Brazilian association for the study of obesity and metabolic syndrome (ABESO-2022).
Pepe RB, Lottenberg AM, Fujiwara CTH, Beyruti M, Cintra DE, Machado RM, Rodrigues A, Jensen NSO, Caldas APS, Fernandes AE, Rossoni C, Mattos F, Motarelli JHF, Bressan J, Saldanha J, Beda LMM, Lavrador MSF, Del Bosco M, Cruz P, Correia PE, Maximino P, Pereira S, Faria SL, Piovacari SMF. Pepe RB, et al. Diabetol Metab Syndr. 2023 Jun 9;15(1):124. doi: 10.1186/s13098-023-01037-6. Diabetol Metab Syndr. 2023. PMID: 37296485 Free PMC article. Review. - The controversial role of the vagus nerve in mediating ghrelin's actions: gut feelings and beyond.
Perelló M, Cornejo MP, De Francesco PN, Fernandez G, Gautron L, Valdivia LS. Perelló M, et al. IBRO Neurosci Rep. 2022 Mar 12;12:228-239. doi: 10.1016/j.ibneur.2022.03.003. eCollection 2022 Jun. IBRO Neurosci Rep. 2022. PMID: 35746965 Free PMC article. Review. - Both high fat and high carbohydrate diets impair vagus nerve signaling of satiety.
Loper H, Leinen M, Bassoff L, Sample J, Romero-Ortega M, Gustafson KJ, Taylor DM, Schiefer MA. Loper H, et al. Sci Rep. 2021 May 17;11(1):10394. doi: 10.1038/s41598-021-89465-0. Sci Rep. 2021. PMID: 34001925 Free PMC article. - Position Statement on Fat Consumption and Cardiovascular Health - 2021.
Izar MCO, Lottenberg AM, Giraldez VZR, Santos Filho RDD, Machado RM, Bertolami A, Assad MHV, Saraiva JFK, Faludi AA, Moreira ASB, Geloneze B, Magnoni CD, Scherr C, Amaral CK, Araújo DB, Cintra DEC, Nakandakare ER, Fonseca FAH, Mota ICP, Santos JED, Kato JT, Beda LMM, Vieira LP, Bertolami MC, Rogero MM, Lavrador MSF, Nakasato M, Damasceno NRT, Alves RJ, Lara RS, Costa RP, Machado VA. Izar MCO, et al. Arq Bras Cardiol. 2021 Jan;116(1):160-212. doi: 10.36660/abc.20201340. Arq Bras Cardiol. 2021. PMID: 33566983 Free PMC article. English, Portuguese. No abstract available.
References
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418: 650–654, 2002. - PubMed
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol 290: G1289–G1297, 2006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical