Theiler's virus infection induces TLR3-dependent upregulation of TLR2 critical for proinflammatory cytokine production - PubMed (original) (raw)
Theiler's virus infection induces TLR3-dependent upregulation of TLR2 critical for proinflammatory cytokine production
Eui Young So et al. Glia. 2009.
Abstract
Theiler's murine encephalomyelitis virus (TMEV) infection directly induces many proinflammatory genes, including type I interferon (IFN) and a variety of cytokine genes. These virus-induced cytokines are a critical factor in developing TMEV-induced demyelinating disease. We have previously reported that the major activation signal for the cytokine genes is mediated via TLR3. In this study, we describe that TLR2 is upregulated via TLR3 signal and cooperatively participates in the expression of IL-6, IL-1beta, CCL2, and CCL5 genes following TMEV infection. The expression of these genes was significantly impaired in both TLR2-deficient and TLR3-deficient primary astrocytes. However, the induction of type I IFNs was not affected by TLR2 deficiency in the primary cells. TMEV infection led to TLR2-mediated NF-kappaB activation, but not IRF3 or IRF7 activation, critical for type I IFN production. More importantly, TLR3 was required for TMEV-induced early TLR2 upregulation in primary astrocytes leading to the production of TLR2-dependent cytokines such as IL-6. Interestingly, soluble factor(s) produced via TLR2/3-dependent signals appears to be partially associated with the downstream cytokine production. These results indicate that TMEV utilizes TLR3-induced TLR2 to induce inflammatory cytokines, which are critical to the development of immune-mediated demyelinating disease.
Figures
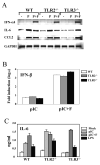
Fig. 1. Primary mouse astrocytes were prepared from WT, TLR2-/- or TLR3-/- mice
(A) Astrocytes were treated with poly (I-C) (50 μg/ml, P) or poly (I-C) complexed with Fugene6 reagent (3 μl/μg poly I:C, P+F). Cells were also stimulated with LPS (5 μg/ml) or TLR4 ligand as controls. After 18 h of stimulation, gene expression levels were analyzed with RT-PCR. (B) IFN-β RNA levels were assessed using real-time PCR, and the results were presented as the fold-induction compared with uninfected cells. (C) IL-6 levels in culture supernatants were assessed with ELISA. Data shown are representative of two independent experiments.
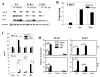
Fig. 2. IL-6 and CCL5 gene expression induced by TMEV is impaired in TLR2-/- astrocytes
(A) Primary mouse astrocytes prepared from WT, TLR2-/- and TLR3-/- mice were infected with TMEV (10 MOI) or treated with LTA (10 μg/ml) and poly(I-C) (pIC, 50 μg/ml) for 6 h. (B) The indicated gene expression was analyzed using RT-PCR. TMEV RNA levels at different times (6, 12, 20 h) in virus infected (20 MOI) cells were also assessed by real-time PCR. Quantitative expression of gene was calculated after normalization with GAPDH expression. (C) IFN-α, IL-6 levels in the supernatants from WT, TLR2-/- or TLR3-/- astrocytes after a 18 h-exposure to TMEV (10 MOI), LTA (10 μg/ml) or pIC (50 μg/ml) were assessed using ELISA. (D) Primary mouse astrocytes were prepared from WT, TLR2-/- and TLR3-/- mice. Cells were left untreated or treated with LTA (5 μg/ml) and poly(I-C) (50 μg/ml) for 6 or 18 h. TLR2 and TLR3 mRNA levels were determined using real-time PCR. The results are representative of 2-3 similar experiments. *, p<0.05 and **, p<0.01.
Fig. 3. TLR2 is important for maximal production of IL-6 in primary mouse astrocytes
Primary mouse astrocytes were isolated from WT and TLR2-/- (A), or TLR4-/- (C) mice. Total RNAs from cells infected with TMEV were subjected to RT-PCR to determine IFN-α4, IFN-β, IL-6, and CCL5 mRNA levels.
Fig. 4. TMEV infection induces TLR2-dependent NF-κB activation, but not IRF3 or IRF7
Parental HEK 293 and HEK 293 cells stably expressing TLR2 or TLR3 were transfected with luciferase reporter gene containing NF-κB-binding sequence (A and C), or with GAL4-IRF7 and UAS(GAL)-reporter plasmid (B), in conjunction with pRL-TK luciferase gene. After 24 h, cells were treated with TMEV, UV-inactivated TMEV, LTA (10 μg/ml) or poly (I-C) (50 μg/ml) for another 24 h. The luciferase activity was determined by fold induction in stimulated cells, compared to the basal level in media-treated cells. The data represent the means of three independent experiments. *, p<0.05 and **, p<0.01.
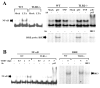
Fig. 5. TLR2 facilitates a maximal NF-κB activation during TMEV infection in astrocytes
(A) Primary astrocytes from WT or TLR2-/- mice were treated with LTA (5 μg/ml), poly I-C (pIC, 50 μg/ml), or TNF-α (5 ng/ml) for 1 h. (B) Some cells were infected with TMEV (10 MOI) for various time periods. Nuclear extracts from these cells were incubated with oligonucleotide containing the consensus NF-κB binding sequence or ISRE sequence. Some nuclear extracts were incubated with anti-p65 or anti-IRF3 antibody before incubation with radio-labeled probe. These mixed samples were subjected to electrophoretic mobility shift assay (EMSA).
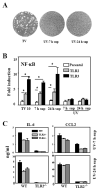
Fig. 6. IL-6 and CCL5 gene activation by TMEV in astrocytes is dependent on the TLR3-mediated upregulated TLR2 expression
(A) Three different stable C8D1A murine astrocyte lines were generated by transfection with siRNA vector containing insert specific for TLR2, TLR3 or none. At 5 h after TMEV infection, the expression levels of genes were determined using RT-PCR. TLR3 expression levels were assessed by real-time PCR. Fold induction of TLR3 was calculated based on differences in the expression between mock- and TMEV-infected samples. (B) Surface TLR2 expression levels on primary astrocytes from WT or TLR3-/- mice infected with TMEV for 3, 7 or 24 h were measured by flow cytometry. ΔMFI (MFI of infected cells – MFI of uninfected cells) was shown as the mean ±SD of two independent experiments. (C) TLR3-/- astrocytes were transfected with 5 μg of mouse TLR2 plasmid or vector alone. Forty-eight hours later, the cells were infected with TMEV (2 or 10 MOI) for 6 h. Their relative cytokine gene expression levels were determined using real-time PCR. Data are presented as the fold-induction in mRNA expression compared with uninfected cells. **, p<0.01 and *, p<0.05 by Student's t test.
Fig. 7. Factors produced by TMEV-infected astrocytes via TLR2/3 promote TLR2-dependent IL-6 production
The supernatants were harvested from wild-type (WT-TV), TLR2-/- (TLR2-/-TV), or TLR3-/- (TLR3-/-TV) astrocytes at 7 h post-infection (7 h sup), and then cells were washed, re-cultured with fresh media for another 17 h to collect additional supernatants (24 h sup). (A) The lack of infectious virus in UV-irradiated (1 h) culture supernatants of wild-type astrocytes was verified by plaque assay on BHK cells and (B) NF-κB activation in HEK293 cells expressing TLR2 or TLR3 as described in Fig 4. (C) UV-inactivated supernatants were added to WT or TLR2-/- astrocytes. After 3 h, cells were washed and re-cultured with fresh media for another 21 h. The supernatants were then assessed for IL-6 and CCL2 levels using ELISA. The results represent the mean of three independent experiments. *p<0.05 by Student's t test.
Similar articles
- TLR3 signaling is either protective or pathogenic for the development of Theiler's virus-induced demyelinating disease depending on the time of viral infection.
Jin YH, Kaneyama T, Kang MH, Kang HS, Koh CS, Kim BS. Jin YH, et al. J Neuroinflammation. 2011 Dec 21;8:178. doi: 10.1186/1742-2094-8-178. J Neuroinflammation. 2011. PMID: 22189096 Free PMC article. - Excessive Innate Immunity Steers Pathogenic Adaptive Immunity in the Development of Theiler's Virus-Induced Demyelinating Disease.
Kim BS. Kim BS. Int J Mol Sci. 2021 May 17;22(10):5254. doi: 10.3390/ijms22105254. Int J Mol Sci. 2021. PMID: 34067536 Free PMC article. Review. - Critical role of TLR activation in viral replication, persistence, and pathogenicity of Theiler's virus.
Kim BS. Kim BS. Front Immunol. 2023 Apr 20;14:1167972. doi: 10.3389/fimmu.2023.1167972. eCollection 2023. Front Immunol. 2023. PMID: 37153539 Free PMC article. Review.
Cited by
- Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function.
Hou W, Jin YH, Kang HS, Kim BS. Hou W, et al. J Virol. 2014 Aug;88(15):8479-89. doi: 10.1128/JVI.00724-14. Epub 2014 May 14. J Virol. 2014. PMID: 24829345 Free PMC article. - Activation of innate immune responses in the central nervous system during reovirus myelitis.
Schittone SA, Dionne KR, Tyler KL, Clarke P. Schittone SA, et al. J Virol. 2012 Aug;86(15):8107-18. doi: 10.1128/JVI.00171-12. Epub 2012 May 23. J Virol. 2012. PMID: 22623770 Free PMC article. - Modeling multiple sclerosis in laboratory animals.
Schreiner B, Heppner FL, Becher B. Schreiner B, et al. Semin Immunopathol. 2009 Nov;31(4):479-95. doi: 10.1007/s00281-009-0181-4. Epub 2009 Oct 3. Semin Immunopathol. 2009. PMID: 19802608 Review. - Transcriptomic profile of host response in Japanese encephalitis virus infection.
Gupta N, Rao PV. Gupta N, et al. Virol J. 2011 Mar 4;8:92. doi: 10.1186/1743-422X-8-92. Virol J. 2011. PMID: 21371334 Free PMC article. - TLR3 signaling is either protective or pathogenic for the development of Theiler's virus-induced demyelinating disease depending on the time of viral infection.
Jin YH, Kaneyama T, Kang MH, Kang HS, Koh CS, Kim BS. Jin YH, et al. J Neuroinflammation. 2011 Dec 21;8:178. doi: 10.1186/1742-2094-8-178. J Neuroinflammation. 2011. PMID: 22189096 Free PMC article.
References
- Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. - PubMed
- Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. - PubMed
- Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 2005;175:4189–93. - PubMed
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS028752/NS/NINDS NIH HHS/United States
- R01 NS033008/NS/NINDS NIH HHS/United States
- R01 NS33008/NS/NINDS NIH HHS/United States
- R01 NS28752/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases