Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites - PubMed (original) (raw)
Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites
Margarita Cabrera et al. Mol Biol Cell. 2009 Apr.
Abstract
Membrane fusion depends on multisubunit tethering factors such as the vacuolar HOPS complex. We previously showed that the vacuolar casein kinase Yck3 regulates vacuole biogenesis via phosphorylation of the HOPS subunit Vps41. Here, we link the identified Vps41 phosphorylation site to HOPS function at the endosome-vacuole fusion site. The nonphosphorylated Vps41 mutant (Vps41 S-A) accumulates together with other HOPS subunits on punctate structures proximal to the vacuole that expand in a class E mutant background and that correspond to in vivo fusion sites. Ultrastructural analysis of this mutant confirmed the presence of tubular endosomal structures close to the vacuole. In contrast, Vps41 with a phosphomimetic mutation (Vps41 S-D) is mislocalized and leads to multilobed vacuoles, indicative of a fusion defect. These two phenotypes can be rescued by overproduction of the vacuolar Rab Ypt7, revealing that both Ypt7 and Yck3-mediated phosphorylation modulate the Vps41 localization to the endosome-vacuole junction. Our data suggest that Vps41 phosphorylation fine-tunes the organization of vacuole fusion sites and provide evidence for a fusion "hot spot" on the vacuole limiting membrane.
Figures
Figure 1.
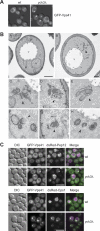
EM analysis of _yck3_Δ cells. (A) Localization of GFP-tagged Vps41. The SEY6210 wild-type and _yck3_Δ cells expressing N-terminally GFP-tagged Vps41 were analyzed by fluorescence microscopy. Overlays between DIC and GFP images are shown. (B) The SEY6210 GFP-VPS41 (A) and SEY6210 _GFP-VPS41 yck3_Δ (B–H) strains were grown to exponential phase and embedded in Spurr's resin as described in Materials and Methods. Arrowheads in B–H point to the anomalous membranous conformations observed in the preparations. C–E highlight the large membranous conformations frequently found adjacent to the vacuole, whereas panels F to H feature the cytoplasmic structures that in most of the cases are circular. N, nucleus; V, vacuole; M, mitochondrion; CW, cell wall; ER, endoplasmic reticulum; PM, plasma membrane. Black bar, 500 nm; white bar 200 nm. (C) Colocalization of GFP-Vps41 with endosomal markers. N-terminally GFP-tagged Vps41 and dsRed-tagged Pep12 or Cps1 were visualized by fluorescence microscopy in SEY6210 wild-type and _yck3_Δ cells. Bar, 10 μm.
Figure 2.
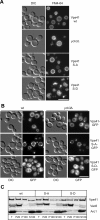
Identification of the Vps41 phosphorylation site. (A) Phosphorylation of Vps41 occurs on membranes. Yeast cells were separated by subcellular fractionation into a low-speed (P4), a medium-speed (P13), and a high-speed pellet (P100). Pellets and the corresponding supernatants (S4, S13, and S100) were incubated for 60 min in the absence or presence of ATP and analyzed by SDS-PAGE and Western blotting using an anti-Vps41 antiserum. (B) Proteins influencing Vps41 phosphorylation. Membranes (P13) obtained from the indicated yeast deletion mutants were incubated without and with ATP and analyzed as described in A. In the Yck3 _cys_Δ, the C-terminal CCCFCC sequence has been removed, in the Yck3 NTD (amino acids 1-333), only the N-terminal domain (NTD) of Yck3 is expressed. (C) Potential casein kinase phosphorylation sites in Vps41. The Yck3 target sequence is highlighted. Sites were identified using the online ProSite software (Expasy,
). (D) Screening of Vps41 mutants. Cells expressing the indicated Vps41 variants were treated and analyzed as described in A. (E) Localization of GFP-tagged Vps41 mutants. Wild-type and the mutant form of Vps41 were C-terminally GFP-tagged and expressed in cells. The resulting strains were incubated with FM4-64 to label vacuoles and analyzed by fluorescence microscopy. Bar, 10 μm. (F) Analysis of the Vps41 phosphorylation site. Membrane fractions of Vps41 alanine mutants were analyzed after ATP incubation as described in A.
Figure 3.
Phosphorylation of Vps41 modulates its localization and function. (A) Vacuole morphology in Vps41 mutants. Strains expressing the indicated Vps41 mutants were incubated with FM4-64 and analyzed by DIC optics or by fluorescence microscopy. Vps41 S-A carries the S367, 368, 371, 372A point mutations, whereas Vps41 S-D contains the comparable phosphomimetic D mutations. Bar, 10 μm. (B) Localization of Vps41 alanine and aspartate mutants in wild-type and _yck3_Δ backgrounds. C-terminal GFP-tagged Vps41 was followed by fluorescence microscopy. Bar, 10 μm. (C) Subcellular fractionation of wild-type and Vps41 mutants. Cells expressing C-terminal GFP-tagged Vps41 wild-type or mutant forms were lysed and separated by centrifugation (20,000 × g for 15 min at 4°C) into pellet (P20) and supernatant fractions. The centrifugation of supernatant (100,000 × g for 1 h at 4°C) was performed to isolate pellet (P100) and supernatant (S100) fractions. Proteins within each fraction were analyzed by SDS-PAGE and Western blotting using antibodies against Vps41, Vac8, and the cytosolic protein marker Arc1. T = 50% of total protein used for each separation.
Figure 4.
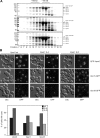
Effect of Vps41 mutations on HOPS complex assembly and on the localization of various fusion factors. (A) HOPS complex assembly. TAP-tagged Vps41 protein was purified from the indicated yeast strains by using IgG beads, natively eluted by TEV cleavage and the eluted proteins were separated on a Superose 6 column. Proteins in each fraction were TCA precipitated and analyzed by SDS-PAGE and Coomassie staining. CbP = calmodulin binding peptide. (B) Localization of tethers in Vps41 wild-type and mutant cells. N-terminally GFP-tagged Vam6 and C-terminally GFP-tagged Vps11 and Vps18 were visualized by fluorescence microscopy. Bar, 10 μm. Cells (200) were counted to statistically analyze the distribution of the GFP signals.
Figure 5.
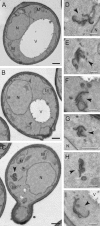
EM analysis of Vps41 mutant cells. The SEY6210 _vam2_Δ _NOP1_pr-VPS41 wild-type (A), SEY6210 _vam2_Δ _NOP1_pr-VPS41 S-D (B), and SEY6210 _vam2_Δ _NOP1_pr-VPS41 S-A (C–I) strains were grown to exponential phase and embedded in Spurr's resin as described in Materials and Methods. Arrowheads in C–I highlight the clusters of membranes observed in cells expressing Vps41 S-A. N, nucleus; V, vacuole; M, mitochondrion; Black bar, 500 nm; white bar, 200 nm.
Figure 6.
Nonphosphorylated Vps41 and Vam6 are enriched at vacuole fusion sites. (A) Localization of Vps41-GFP in _vps27_Δ strain. SEY6210 cells expressing C-terminal GFP-tagged wild-type and mutant (S-A) Vps41 were labeled with FM4-64 and analyzed by fluorescence microscopy. Where indicated, VPS27 was deleted. Bar, 10 μm. (B) Localization of GFP-Vam6 in the _vps27_Δ strain. SEY6210 wild-type and _vps27_Δ cells expressing N-terminal GFP-tagged Vam6 in the respective Vps41 background were incubated with FM4-64 and analyzed by fluorescence microscopy. Bar, 10 μm. (C) In vivo fusion of vacuoles after relieve from high salt concentration stress. Cells expressing the Vps41 S-A mutant and the N-terminally GFP-tagged Vam6 fusion were incubated with 0.4 M NaCl for 30 min, followed by a wash with YPD medium and grown for the indicated times. Localization of Vam6 and vacuole morphology were monitored by fluorescence microscopy. Bar, 10 μm.
Figure 7.
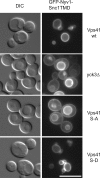
The AP-3 pathway is impaired in the Vps41 S-A mutant. Localization of GFP-Nyv1-Snc1TMD in Vps41 wild-type and mutant cells. Cells expressing GFP-tagged Nyv1-Snc1TMD in the indicated Vps41 backgrounds were visualized by fluorescence microscopy. Bar, 10 μm.
Figure 8.
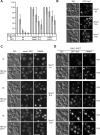
Vps41 phosphorylation is linked to Rab Ypt7 cycle. (A) Sensitivity of vacuole fusion to Gyp1. Standard fusion reactions containing BJ and DKY vacuoles from the indicated strains were incubated for 90 min with or without ATP and increasing amounts of Gyp1. Fusion activity was determined as described in Materials and Methods. Fusion is set to 100% for untreated cells. OD400 was 0.54 ± 0.07 for wild-type, 0.41 ± 0.08 for _yck3_Δ cells, and for vacuoles expressing Vps41 S-A, 0.47 ± 0.07. (B) GFP-Ypt7 localization in the Vps41 mutants. Strains carrying N-terminal GFP-tagged Ypt7 were visualized by DIC and fluorescence microscopy. Bar, 10 μm. (C) Effect of Ypt7 overproduction on Vps41 localization. Cells expressing Ypt7 under the TEF promoter and C-terminally GFP-tagged Vps41 or mutants were analyzed by fluorescence microscopy. (D) Ypt7 inactivation triggered by Gyp7 overproduction. Diploid strains containing GFP-tagged Ypt7 and Vps41 wt or the indicated mutants were grown in YPD or YPG to induce Gyp7-overproduction. Vacuoles were stained with FM4-64 and visualized in the fluorescence microscope. Bar, 10 μm.
Similar articles
- Phosphorylation of a membrane curvature-sensing motif switches function of the HOPS subunit Vps41 in membrane tethering.
Cabrera M, Langemeyer L, Mari M, Rethmeier R, Orban I, Perz A, Bröcker C, Griffith J, Klose D, Steinhoff HJ, Reggiori F, Engelbrecht-Vandré S, Ungermann C. Cabrera M, et al. J Cell Biol. 2010 Nov 15;191(4):845-59. doi: 10.1083/jcb.201004092. J Cell Biol. 2010. PMID: 21079247 Free PMC article. - Vps41 functions as a molecular ruler for HOPS tethering complex-mediated membrane fusion.
König C, Shvarev D, Gao J, Haar E, Susan N, Auffarth K, Langemeyer L, Moeller A, Ungermann C. König C, et al. J Cell Sci. 2025 Apr 15;138(8):jcs263788. doi: 10.1242/jcs.263788. Epub 2025 Apr 25. J Cell Sci. 2025. PMID: 40159992 - Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase.
Brett CL, Plemel RL, Lobingier BT, Vignali M, Fields S, Merz AJ. Brett CL, et al. J Cell Biol. 2008 Sep 22;182(6):1141-51. doi: 10.1083/jcb.200801001. J Cell Biol. 2008. PMID: 18809726 Free PMC article. - Membrane dynamics and fusion at late endosomes and vacuoles--Rab regulation, multisubunit tethering complexes and SNAREs.
Epp N, Rethmeier R, Krämer L, Ungermann C. Epp N, et al. Eur J Cell Biol. 2011 Sep;90(9):779-85. doi: 10.1016/j.ejcb.2011.04.007. Epub 2011 Jun 16. Eur J Cell Biol. 2011. PMID: 21683469 Review. - Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles.
Wickner W. Wickner W. Annu Rev Cell Dev Biol. 2010;26:115-36. doi: 10.1146/annurev-cellbio-100109-104131. Annu Rev Cell Dev Biol. 2010. PMID: 20521906 Review.
Cited by
- Sphingolipids containing very long-chain fatty acids regulate Ypt7 function during the tethering stage of vacuole fusion.
Zhang C, Calderin JD, Hurst LR, Gokbayrak ZD, Hrabak MR, Balutowski A, Rivera-Kohr DA, Kazmirchuk TDD, Brett CL, Fratti RA. Zhang C, et al. J Biol Chem. 2024 Nov;300(11):107808. doi: 10.1016/j.jbc.2024.107808. Epub 2024 Sep 21. J Biol Chem. 2024. PMID: 39307308 Free PMC article. - Cargo Release from Myosin V Requires the Convergence of Parallel Pathways that Phosphorylate and Ubiquitylate the Cargo Adaptor.
Wong S, Hepowit NL, Port SA, Yau RG, Peng Y, Azad N, Habib A, Harpaz N, Schuldiner M, Hughson FM, MacGurn JA, Weisman LS. Wong S, et al. Curr Biol. 2020 Nov 16;30(22):4399-4412.e7. doi: 10.1016/j.cub.2020.08.062. Epub 2020 Sep 10. Curr Biol. 2020. PMID: 32916113 Free PMC article. - Class IA phosphatidylinositol 3-kinase p110α regulates phagosome maturation.
Thi EP, Lambertz U, Reiner NE. Thi EP, et al. PLoS One. 2012;7(8):e43668. doi: 10.1371/journal.pone.0043668. Epub 2012 Aug 22. PLoS One. 2012. PMID: 22928013 Free PMC article. - Arabidopsis HOPS subunit VPS41 carries out plant-specific roles in vacuolar transport and vegetative growth.
Jiang D, He Y, Zhou X, Cao Z, Pang L, Zhong S, Jiang L, Li R. Jiang D, et al. Plant Physiol. 2022 Jun 27;189(3):1416-1434. doi: 10.1093/plphys/kiac167. Plant Physiol. 2022. PMID: 35417008 Free PMC article. - A Rab prenyl membrane-anchor allows effector recognition to be regulated by guanine nucleotide.
Lee M, Wickner W, Song H. Lee M, et al. Proc Natl Acad Sci U S A. 2020 Apr 7;117(14):7739-7744. doi: 10.1073/pnas.2000923117. Epub 2020 Mar 25. Proc Natl Acad Sci U S A. 2020. PMID: 32213587 Free PMC article.
References
- Albert S., Gallwitz D. Two new members of a family of Ypt/Rab GTPase activating proteins. Promiscuity of substrate recognition. J. Biol. Chem. 1999;274:33186–33189. - PubMed
- Bos J. L., Rehmann H., Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases