Top-down activation of shape-specific population codes in visual cortex during mental imagery - PubMed (original) (raw)
Comparative Study
Top-down activation of shape-specific population codes in visual cortex during mental imagery
Mark Stokes et al. J Neurosci. 2009.
Abstract
Visual imagery is mediated via top-down activation of visual cortex. Similar to stimulus-driven perception, the neural configurations associated with visual imagery are differentiated according to content. For example, imagining faces or places differentially activates visual areas associated with perception of actual face or place stimuli. However, while top-down activation of topographically specific visual areas during visual imagery is well established, the extent to which internally generated visual activity resembles the fine-scale population coding responsible for stimulus-driven perception remains unknown. Here, we sought to determine whether top-down mechanisms can selectively activate perceptual representations coded across spatially overlapping neural populations. We explored the precision of top-down activation of perceptual representations using neural pattern classification to identify activation patterns associated with imagery of distinct letter stimuli. Pattern analysis of the neural population observed within high-level visual cortex, including lateral occipital complex, revealed that imagery activates the same neural representations that are activated by corresponding visual stimulation. We conclude that visual imagery is mediated via top-down activation of functionally distinct, yet spatially overlapping population codes for high-level visual representations.
Figures
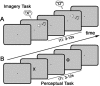
Figure 1.
The experimental design. A, During the imagery task, participants were cued (via either a high or low pitch auditory tone) on a trial-by-trial basis to imagine either X or O. The intertrial interval (ITI) was randomized between 3 and 12 s. B, During the perceptual task, participants were instructed to watch a series of visually presented letter stimuli (X or O; duration 250 ms, ITI 3–12 s) and press the same button following the presentation of each stimulus.
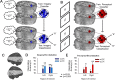
Figure 2.
Activation results for the conventional univariate analyses. A, During the imagery task, comparisons against baseline confirmed relative increases in activity distributed throughout visual cortex, including cuneus (Cu), lingual gyrus (LiG) and middle temporal gyrus (MTG). Event-related increases were also observed within superior temporal sulcus/Heschl's gyrus (STG/HG). B, During the perceptual task, a similar network of brain areas was also activated, with peaks in inferior occipital gyrus (IOG), middle occipital gyrus (MOG), middle temporal gyrus (MTG) and fusiform gyrus (FG). Beyond visual cortex, precentral gyrus (PrG) was also active during the perceptual task. All activation maps are corrected for multiple comparisons (p FDR < 0.05). Shaded brain areas were beyond the functional data acquisition field of view within at least one subject, and therefore not included in the group analyses. The calcarine sulcus (CcS) is indicated by the black line superimposed over coronal slices.
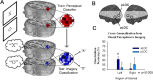
Figure 3.
A, B, Train-test neural classification was used to index population coding during visual imagery (A) and perception (B). C, Pattern analyses were performed in aLOC and pLOC of both hemispheres, shown here on a rendered brain surface. Shading indicates the regions beyond the functional data acquisition field of view. D, Differential population coding of the two alternative states of imagery was observed within all subregions of area LOC. E, During the perceptual task, there was a trend toward above-chance discrimination across all subregions of LOC, however, perceptual classification was only significantly above-chance within the left and right pLOC. Error bars represent ± 1 SEM, calculated across participants.
Figure 4.
Cross-generalization MVPA reveals the coding similarity between visual imagery and perception. A, First, a perceptual classifier was trained to discriminate between patterns of visual activity associated with alternative perceptual conditions during the perceptual task. Second, the perceptual classifier was used to predict the imagery state associated with visual activity observed during the imagery task. B, Cross-generalization pattern analyses were performed in aLOC and pLOC of both hemispheres, shown here on a rendered brain surface with shading to indicate the regions beyond the functional data acquisition field of view. C, Accurate cross-generalization between perception and imagery confirms significant similarity between respective population coding in the left aLOC. Error bars represent ± 1 SEM, calculated across participants.
Figure 5.
Population coding during visual imagery and perception. A, Searchlight MVPA revealed differential population coding of the two alternative states of imagery within the high-level visual areas, including inferior occipital gyrus (IOG), middle occipital gyrus (MOG), fusiform gyrus (FG) and middle temporal gyrus (MTG). Searchlight analyses also identified discriminative clusters in superior temporal gyrus/Heschl's gyrus (STG/HG), and anterior insula/frontal operculum (aI/fO). B, Searchlight MVPA of visual perception revealed a similar network of discriminative clusters, with the exception of STG/HG. Classification accuracies are shown for voxels that survived correction for multiple comparisons (FDR <5%), and shading indicates brain areas that were beyond the functional data acquisition field of view within at least one subject, and therefore not included in the group analyses. For reference, anterior lateral occipital complex (aLOC), posterior lateral occipital complex (pLOC) and calcarine sulcus (CcS) are superimposed in black.
Similar articles
- Reconstructing imagined letters from early visual cortex reveals tight topographic correspondence between visual mental imagery and perception.
Senden M, Emmerling TC, van Hoof R, Frost MA, Goebel R. Senden M, et al. Brain Struct Funct. 2019 Apr;224(3):1167-1183. doi: 10.1007/s00429-019-01828-6. Epub 2019 Jan 14. Brain Struct Funct. 2019. PMID: 30637491 Free PMC article. - Distinct Top-down and Bottom-up Brain Connectivity During Visual Perception and Imagery.
Dijkstra N, Zeidman P, Ondobaka S, van Gerven MAJ, Friston K. Dijkstra N, et al. Sci Rep. 2017 Jul 18;7(1):5677. doi: 10.1038/s41598-017-05888-8. Sci Rep. 2017. PMID: 28720781 Free PMC article. - Imagery for shapes activates position-invariant representations in human visual cortex.
Stokes M, Saraiva A, Rohenkohl G, Nobre AC. Stokes M, et al. Neuroimage. 2011 Jun 1;56(3):1540-5. doi: 10.1016/j.neuroimage.2011.02.071. Epub 2011 Mar 3. Neuroimage. 2011. PMID: 21376815 - Shared Neural Mechanisms of Visual Perception and Imagery.
Dijkstra N, Bosch SE, van Gerven MAJ. Dijkstra N, et al. Trends Cogn Sci. 2019 May;23(5):423-434. doi: 10.1016/j.tics.2019.02.004. Epub 2019 Mar 12. Trends Cogn Sci. 2019. PMID: 30876729 Review. - The relationship between visual perception and visual mental imagery: a reappraisal of the neuropsychological evidence.
Bartolomeo P. Bartolomeo P. Cortex. 2002 Jun;38(3):357-78. doi: 10.1016/s0010-9452(08)70665-8. Cortex. 2002. PMID: 12146661 Review.
Cited by
- Towards an intuitive communication-BCI: decoding visually imagined characters from the early visual cortex using high-field fMRI.
van den Boom MA, Vansteensel MJ, Koppeschaar MI, Raemaekers MAH, Ramsey NF. van den Boom MA, et al. Biomed Phys Eng Express. 2019 Aug 2;5(5):055001. doi: 10.1088/2057-1976/ab302c. eCollection 2019 Aug. Biomed Phys Eng Express. 2019. PMID: 32983573 Free PMC article. - Improving visual perception through neurofeedback.
Scharnowski F, Hutton C, Josephs O, Weiskopf N, Rees G. Scharnowski F, et al. J Neurosci. 2012 Dec 5;32(49):17830-41. doi: 10.1523/JNEUROSCI.6334-11.2012. J Neurosci. 2012. PMID: 23223302 Free PMC article. - Reconstructing imagined letters from early visual cortex reveals tight topographic correspondence between visual mental imagery and perception.
Senden M, Emmerling TC, van Hoof R, Frost MA, Goebel R. Senden M, et al. Brain Struct Funct. 2019 Apr;224(3):1167-1183. doi: 10.1007/s00429-019-01828-6. Epub 2019 Jan 14. Brain Struct Funct. 2019. PMID: 30637491 Free PMC article. - Decoding action intentions from preparatory brain activity in human parieto-frontal networks.
Gallivan JP, McLean DA, Valyear KF, Pettypiece CE, Culham JC. Gallivan JP, et al. J Neurosci. 2011 Jun 29;31(26):9599-610. doi: 10.1523/JNEUROSCI.0080-11.2011. J Neurosci. 2011. PMID: 21715625 Free PMC article. - Multi-Voxel Decoding and the Topography of Maintained Information During Visual Working Memory.
Lee SH, Baker CI. Lee SH, et al. Front Syst Neurosci. 2016 Feb 15;10:2. doi: 10.3389/fnsys.2016.00002. eCollection 2016. Front Syst Neurosci. 2016. PMID: 26912997 Free PMC article. Review.
References
- Amedi A, Malach R, Hendler T, Peled S, Zohary E. Visuo-haptic object-related activation in the ventral visual pathway. Nat Neurosci. 2001;4:324–330. - PubMed
- Baars BJ, Franklin S. How conscious experience and working memory interact. Trends Cogn Sci. 2003;7:166–172. - PubMed
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 1999;2:671–676. - PubMed
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources