Structure and function of the 5'-->3' exoribonuclease Rat1 and its activating partner Rai1 - PubMed (original) (raw)
Structure and function of the 5'-->3' exoribonuclease Rat1 and its activating partner Rai1
Song Xiang et al. Nature. 2009.
Abstract
The 5'-->3' exoribonucleases (XRNs) comprise a large family of conserved enzymes in eukaryotes with crucial functions in RNA metabolism and RNA interference. XRN2, or Rat1 in yeast, functions primarily in the nucleus and also has an important role in transcription termination by RNA polymerase II (refs 7-14). Rat1 exoribonuclease activity is stimulated by the protein Rai1 (refs 15, 16). Here we report the crystal structure at 2.2 A resolution of Schizosaccharomyces pombe Rat1 in complex with Rai1, as well as the structures of Rai1 and its murine homologue Dom3Z alone at 2.0 A resolution. The structures reveal the molecular mechanism for the activation of Rat1 by Rai1 and for the exclusive exoribonuclease activity of Rat1. Biochemical studies confirm these observations, and show that Rai1 allows Rat1 to degrade RNAs with stable secondary structure more effectively. There are large differences in the active site landscape of Rat1 compared to related and PIN (PilT N terminus) domain-containing nucleases. Unexpectedly, we identified a large pocket in Rai1 and Dom3Z that contains highly conserved residues, including three acidic side chains that coordinate a divalent cation. Mutagenesis and biochemical studies demonstrate that Rai1 possesses pyrophosphohydrolase activity towards 5' triphosphorylated RNA. Such an activity is important for messenger RNA degradation in bacteria, but this is, to our knowledge, the first demonstration of this activity in eukaryotes and suggests that Rai1/Dom3Z may have additional important functions in RNA metabolism.
Figures
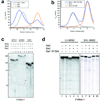
Figure 1. Structure of the Rat1-Rai1 complex
(a). Domain organization of S. pombe Rat1, S. cerevisiae Rat1, human XRN2 and human XRN1. The first conserved region is colored in cyan, the second in magenta, and the linker segment between them in gray. A poorly conserved segment in the C-terminus that is also observed in our structure is shown in yellow. (b). Schematic drawing of the structure of S. pombe Rat1-Rai1 complex. The structure of Rat1 is colored as in panel a, and the structure of Rai1 is in green. The active site of Rat1 is indicated with the red star, and the red arrow points to the opening of the Rai1 active site pocket. A bound divalent cation in the active site of Rai1 is shown as a gray sphere. (c). Molecular surface of the active site region of Rat1, colored as in panel a. (d). Good surface complementarity at the interface between Rat1 and Rai1. Rat1 is shown as a molecular surface, and residues in the interface with Rai1 are colored in light blue and yellow for the first conserved region and the C-terminal segment, respectively. Rai1 is shown as stick models, with carbon atoms in black. All the structure figures were produced with Pymol or Grasp .
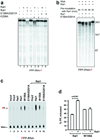
Figure 2. Biochemical and functional characterization of the Rat1-Rai1 interaction
(a). Gel filtration profiles for wild-type Rat1 (full-length) alone, wild-type Rai1 (full-length) alone, and their mixture (with Rai1 present in roughly 2-fold molar excess). (b). Gel filtration profiles for wild-type Rat1 alone, W159A mutant of Rai1 alone, and their mixture. (c). Cleavage of three different 5’ phosphorylated, 3’ labeled RNA substrates by Rat1 and the Rat1-Rai1 complex. The RNA substrate is indicated at the bottom of the figure, with the radiolabeled phosphate group shown in red. (d). Cleavage of two other RNA substrates, each with three MS2 binding sites, by Rat1 and the Rat1-Rai1 complex.
Figure 3. Structure of Rai1
(a). Schematic drawing of the structure of S. pombe Rai1. Strands in the large β-sheet are shown in green, and those in the small β-sheet in cyan. Side chains of some of the conserved residues in the large pocket in the structure are shown in black, and the pocket is highlighted in light pink. A bound divalent cation is shown as a gray sphere. The arrow indicates the interface region with Rat1. (b). Molecular surface of Rai1, showing the large pocket in the structure. (c). Final 2Fo–Fc electron density (in light blue) at 2.2 Å resolution for the divalent cation and its ligands in the large pocket in Rai1, contoured at 1.5σ. Omit Fo–Fc electron density for the cation and the two water molecules is shown in green, contoured at 3σ. (d). Schematic drawing of the detailed interactions between GDP (in light gray) and DOM3Z (side chains in black). (e). Panel d after 90° rotation around the horizontal axis.
Figure 4. Biochemical evidence for pyrophosphohydrolase activity of Rai1
(a). Cleavage of 5’ triphosphorylated, 3’ labeled RNA substrate by Rat1 and the Rat1-Rai1 complex. Rat1 does not exhibit ribonuclease activity towards this RNA in the absence of Rai1. The E199A/D201A mutant does not enable this ribonuclease activity. (b). Pre-incubation of 5’ triphosphorylated RNA with wild-type Rai1, but not E199A/D201A mutant, allows Rat1 to degrade the substrate. (c). Rai1 can release pyrophosphate from RNA with 5’ triphosphate. The assays were carried out in the absence (lanes 1–6) or presence (lanes 7–10) of Rat1. Human Dcp2 decapping protein was used as a negative control. The pyrophosphate marker is indicated on the left and was generated by RNA polymerase during in vitro transcription, and could be clearly distinguished from free phosphate. (d). Quantification of the percent pyrophosphate generated by Rai1 from four independent experiments. The error bars represent standard error of the mean.
Similar articles
- Structural basis of eukaryotic transcription termination by the Rat1 exonuclease complex.
Yanagisawa T, Murayama Y, Ehara H, Goto M, Aoki M, Sekine SI. Yanagisawa T, et al. Nat Commun. 2024 Sep 8;15(1):7854. doi: 10.1038/s41467-024-52157-0. Nat Commun. 2024. PMID: 39245712 Free PMC article. - Structures of 5'-3' Exoribonucleases.
Chang JH, Xiang S, Tong L. Chang JH, et al. Enzymes. 2012;31:115-29. doi: 10.1016/B978-0-12-404740-2.00006-9. Epub 2012 Sep 29. Enzymes. 2012. PMID: 27166443 - A novel 5'-hydroxyl dinucleotide hydrolase activity for the DXO/Rai1 family of enzymes.
Doamekpor SK, Gozdek A, Kwasnik A, Kufel J, Tong L. Doamekpor SK, et al. Nucleic Acids Res. 2020 Jan 10;48(1):349-358. doi: 10.1093/nar/gkz1107. Nucleic Acids Res. 2020. PMID: 31777937 Free PMC article. - XRN 5'→3' exoribonucleases: structure, mechanisms and functions.
Nagarajan VK, Jones CI, Newbury SF, Green PJ. Nagarajan VK, et al. Biochim Biophys Acta. 2013 Jun-Jul;1829(6-7):590-603. doi: 10.1016/j.bbagrm.2013.03.005. Epub 2013 Mar 19. Biochim Biophys Acta. 2013. PMID: 23517755 Free PMC article. Review. - mRNA quality control at the 5' end.
Zhai LT, Xiang S. Zhai LT, et al. J Zhejiang Univ Sci B. 2014 May;15(5):438-43. doi: 10.1631/jzus.B1400070. J Zhejiang Univ Sci B. 2014. PMID: 24793761 Free PMC article. Review.
Cited by
- A mammalian pre-mRNA 5' end capping quality control mechanism and an unexpected link of capping to pre-mRNA processing.
Jiao X, Chang JH, Kilic T, Tong L, Kiledjian M. Jiao X, et al. Mol Cell. 2013 Apr 11;50(1):104-15. doi: 10.1016/j.molcel.2013.02.017. Epub 2013 Mar 21. Mol Cell. 2013. PMID: 23523372 Free PMC article. - How hydrolytic exoribonucleases impact human disease: Two sides of the same story.
Costa SM, Saramago M, Matos RG, Arraiano CM, Viegas SC. Costa SM, et al. FEBS Open Bio. 2023 Jun;13(6):957-974. doi: 10.1002/2211-5463.13392. Epub 2022 Mar 20. FEBS Open Bio. 2023. PMID: 35247037 Free PMC article. Review. - Identification of new homologs of PD-(D/E)XK nucleases by support vector machines trained on data derived from profile-profile alignments.
Laganeckas M, Margelevicius M, Venclovas C. Laganeckas M, et al. Nucleic Acids Res. 2011 Mar;39(4):1187-96. doi: 10.1093/nar/gkq958. Epub 2010 Oct 20. Nucleic Acids Res. 2011. PMID: 20961958 Free PMC article. - Role of the RNA/DNA kinase Grc3 in transcription termination by RNA polymerase I.
Braglia P, Heindl K, Schleiffer A, Martinez J, Proudfoot NJ. Braglia P, et al. EMBO Rep. 2010 Oct;11(10):758-64. doi: 10.1038/embor.2010.130. Epub 2010 Sep 3. EMBO Rep. 2010. PMID: 20814424 Free PMC article. - 2'-O-methylation of the mRNA cap protects RNAs from decapping and degradation by DXO.
Picard-Jean F, Brand C, Tremblay-Létourneau M, Allaire A, Beaudoin MC, Boudreault S, Duval C, Rainville-Sirois J, Robert F, Pelletier J, Geiss BJ, Bisaillon M. Picard-Jean F, et al. PLoS One. 2018 Mar 30;13(3):e0193804. doi: 10.1371/journal.pone.0193804. eCollection 2018. PLoS One. 2018. PMID: 29601584 Free PMC article.
References
- Parker R, Song H. The enzymes and control of eukaryotic mRNA turnover. Nat. Struct. Mol. Biol. 2004;11:121–127. - PubMed
- Newbury SF. Control of mRNA stability in eukaryotes. Biochem. Soc. Trans. 2006;34:30–34. - PubMed
- Bousquet-Antonelli C, Presutti C, Tollervey D. Identification of a regulated pathway for nuclear pre-mRNA turnover. Cell. 2000;102:765–775. - PubMed
- Gatfield D, Izaurralde E. Nonsense-mediated messenger RNA decay is initiated by endonucleolytic cleavage in Drosophila. Nature. 2004;429:575–578. - PubMed
- Gazzani S, Lawrenson T, Woodward C, Headon D, Sablowski R. A link between mRNA turnover and RNA interference in Arabidopsis. Science. 2004;306:1046–1048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM028983/GM/NIGMS NIH HHS/United States
- GM077175/GM/NIGMS NIH HHS/United States
- R01 GM067005/GM/NIGMS NIH HHS/United States
- R01 GM077175/GM/NIGMS NIH HHS/United States
- R01 GM067005-01A2/GM/NIGMS NIH HHS/United States
- P30 EB009998/EB/NIBIB NIH HHS/United States
- GM67005/GM/NIGMS NIH HHS/United States
- R01 GM077175-02/GM/NIGMS NIH HHS/United States
- GM28983/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous