Association of reactive oxygen species levels and radioresistance in cancer stem cells - PubMed (original) (raw)
. 2009 Apr 9;458(7239):780-3.
doi: 10.1038/nature07733.
Robert W Cho, Neethan A Lobo, Tomer Kalisky, Mary Jo Dorie, Angela N Kulp, Dalong Qian, Jessica S Lam, Laurie E Ailles, Manzhi Wong, Benzion Joshua, Michael J Kaplan, Irene Wapnir, Frederick M Dirbas, George Somlo, Carlos Garberoglio, Benjamin Paz, Jeannie Shen, Sean K Lau, Stephen R Quake, J Martin Brown, Irving L Weissman, Michael F Clarke
Affiliations
- PMID: 19194462
- PMCID: PMC2778612
- DOI: 10.1038/nature07733
Association of reactive oxygen species levels and radioresistance in cancer stem cells
Maximilian Diehn et al. Nature. 2009.
Abstract
The metabolism of oxygen, although central to life, produces reactive oxygen species (ROS) that have been implicated in processes as diverse as cancer, cardiovascular disease and ageing. It has recently been shown that central nervous system stem cells and haematopoietic stem cells and early progenitors contain lower levels of ROS than their more mature progeny, and that these differences are critical for maintaining stem cell function. We proposed that epithelial tissue stem cells and their cancer stem cell (CSC) counterparts may also share this property. Here we show that normal mammary epithelial stem cells contain lower concentrations of ROS than their more mature progeny cells. Notably, subsets of CSCs in some human and murine breast tumours contain lower ROS levels than corresponding non-tumorigenic cells (NTCs). Consistent with ROS being critical mediators of ionizing-radiation-induced cell killing, CSCs in these tumours develop less DNA damage and are preferentially spared after irradiation compared to NTCs. Lower ROS levels in CSCs are associated with increased expression of free radical scavenging systems. Pharmacological depletion of ROS scavengers in CSCs markedly decreases their clonogenicity and results in radiosensitization. These results indicate that, similar to normal tissue stem cells, subsets of CSCs in some tumours contain lower ROS levels and enhanced ROS defences compared to their non-tumorigenic progeny, which may contribute to tumour radioresistance.
Figures
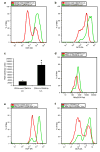
Figure 1. Analysis of ROS levels in normal mammary and breast cancer stem cells and their progeny
a, CD24medCD49fhighLin- mammary cells (mammary stem cell-containing population) and CD24highCD49flowLin- mammary cells (progenitor cell-containing population) were isolated from C57Bl/6J female mice using flow cytometry and intracellular ROS concentrations were measured by DCF-DA staining. b, as in a but using 29S1/SvImJ mice. c, Mean ± s.e.m. for replicates of a and b (n=6; _p_=0.001). d, as in b but using MitoSOX Red instead of DCF-DA. Data shown are representative of two independent experiments. e, CD44+CD24-/lowLin- breast cancer cells (CSC-containing population) and “Not CD44+CD24-/low” Lin- cells (non-tumorigenic population) were isolated from a primary human breast tumor by flow cytometry and ROS levels were analyzed using DCF-DA. f, as in e but using murine Thy1+CD24+Lin- breast cancer cells (CSC-containing population) and “Not Thy1+CD24+” Lin- cells (non-tumorigenic population) isolated from an MMTV-Wnt-1 breast tumor.
Figure 2. Thy1+CD24+Lin- CSC-enriched cells develop less DNA damage after irradiation than non-tumorigenic cells
a, Thy1+CD24+Lin- cells (CSC-enriched population) and “Not Thy1+CD24+” Lin- non-tumorigenic cells were isolated from MMTV-Wnt-1 breast tumors by flow cytometry and irradiated with 10 Gy of IR. DNA damage was measured before irradiation, immediately after irradiation, and 16 hrs later using the alkaline comet assay. Mean of median tail moments ± s.e.m. (n=3; _p_=0.05). b, Using the data from a, the difference in median tail moments between the untreated and “on ice” time points was calculated. Mean ± s.e.m. (n=3; _p_=0.004). c, Thy1+CD24+Lin- cells and “Not Thy1+CD24+” Lin- non-tumorigenic cells from MMTV-Wnt-1 tumors that were processed and collected 15 minutes after being irradiated in vivo with 1 Gy of IR were immunostained for γ-H2AX, a marker of DNA double strand breaks. Mean ± s.e.m. (n=2; _p_=0.04).
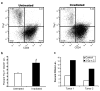
Figure 3. Enrichment of CSCs after in vivo irradiation
a, Breast tumors from MMTV-Wnt-1 mice were irradiated in vivo with 3 × 5 Gy or 5 × 2 Gy. Percentage of Thy1+CD24+Lin- cells in untreated and irradiated tumors were quantified by flow cytometry 72 hours after the last fraction was delivered. b, Mean ± s.e.m. for replicates of a (n=6; _p_=0.008). c, First generation xenografts established from two different primary human head and neck cancers were irradiated in vivo with 2 × 3 Gy. Percentage of CD44+Lin- cells was quantified as above.
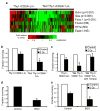
Figure 4. Thy1+CD24+Lin- cells overexpress genes involved in ROS scavenging and pharmacologic modulation of ROS levels affects the radiosensitivity of Thy1+CD24+Lin- and “Not Thy1+CD24+” Lin-cells
a, Single cell qRT-PCR analysis of gene expression in Thy1+CD24+Lin- CSC-enriched cells and “Not Thy1+CD24+” Lin- non-tumorigenic cells. The heatmap displays mean centered CT values. b, Clonogenic survival of Thy1+CD24+Lin- CSC-enriched cells and “Not Thy1+CD24+” Lin- non-tumorigenic cells before and after 2 Gy of ionizing radiation. Mean ± s.e.m. (n=3; _p_=0.001). c, Clonogenic survival of “Not Thy1+CD24+” Lin- non-tumorigenic cells in the presence or absence of the ROS scavenger tempol (10 mM). Mean ± s.e.m. (n=2; _p_=0.03). d, Clonogenic survival of Thy1+CD24+Lin- CSC-enriched cells in the presence or absence of 24 hour pre-treatment with the glutathione synthesis inhibitor L-_S,R_-Buthionine Sulfoximine (BSO, 1 mM). Mean ± s.e.m. (n=3; _p_=0.002). e, Clonogenic survival of Thy1+CD24+Lin- CSC-enriched cells after 3 Gy of ionizing radiation with or without BSO pretreatment. Mean ± s.e.m. (n=3; _p_=0.03).
Similar articles
- A radical bailout strategy for cancer stem cells.
Tothova Z, Gilliland DG. Tothova Z, et al. Cell Stem Cell. 2009 Mar 6;4(3):196-7. doi: 10.1016/j.stem.2009.02.008. Cell Stem Cell. 2009. PMID: 19265655 - Radiation resistance: Cancer stem cells (CSCs) and their enigmatic pro-survival signaling.
Skvortsova I, Debbage P, Kumar V, Skvortsov S. Skvortsova I, et al. Semin Cancer Biol. 2015 Dec;35:39-44. doi: 10.1016/j.semcancer.2015.09.009. Epub 2015 Sep 25. Semin Cancer Biol. 2015. PMID: 26392376 Review. - Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation.
Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, Park HG, Han SI, Kang HS. Lee SY, et al. Mol Cancer. 2017 Jan 30;16(1):10. doi: 10.1186/s12943-016-0577-4. Mol Cancer. 2017. PMID: 28137309 Free PMC article. Review. - Predominant factors influencing reactive oxygen species in cancer stem cells.
Soundararajan L, Warrier S, Dharmarajan A, Bhaskaran N. Soundararajan L, et al. J Cell Biochem. 2024 Jan;125(1):3-21. doi: 10.1002/jcb.30506. Epub 2023 Nov 24. J Cell Biochem. 2024. PMID: 37997702 Review.
Cited by
- The stabilization of yes-associated protein by TGFβ-activated kinase 1 regulates the self-renewal and oncogenesis of gastric cancer stem cells.
Wang G, Sun Q, Zhu H, Bi Y, Zhu H, Xu A. Wang G, et al. J Cell Mol Med. 2021 Jul;25(14):6584-6601. doi: 10.1111/jcmm.16660. Epub 2021 Jun 1. J Cell Mol Med. 2021. PMID: 34075691 Free PMC article. - TRA-1-60+, SSEA-4+, Oct4A+, Nanog+ Clones of Pluripotent Stem Cells in the Embryonal Carcinomas of the Ovaries.
Malecki M, Anderson M, Beauchaine M, Seo S, Tombokan X, Malecki R. Malecki M, et al. J Stem Cell Res Ther. 2012 Nov 18;2(5):130. J Stem Cell Res Ther. 2012. PMID: 23293749 Free PMC article. - Liposome-Imipramine Blue Inhibits Sonic Hedgehog Medulloblastoma In Vivo.
MacDonald TJ, Liu J, Yu B, Malhotra A, Munson J, Park JC, Wang K, Fei B, Bellamkonda R, Arbiser J. MacDonald TJ, et al. Cancers (Basel). 2021 Mar 11;13(6):1220. doi: 10.3390/cancers13061220. Cancers (Basel). 2021. PMID: 33799550 Free PMC article. - Bmi1 confers resistance to oxidative stress on hematopoietic stem cells.
Nakamura S, Oshima M, Yuan J, Saraya A, Miyagi S, Konuma T, Yamazaki S, Osawa M, Nakauchi H, Koseki H, Iwama A. Nakamura S, et al. PLoS One. 2012;7(5):e36209. doi: 10.1371/journal.pone.0036209. Epub 2012 May 11. PLoS One. 2012. PMID: 22606246 Free PMC article. - Opportunities and challenges of radiotherapy for treating cancer.
Schaue D, McBride WH. Schaue D, et al. Nat Rev Clin Oncol. 2015 Sep;12(9):527-40. doi: 10.1038/nrclinonc.2015.120. Epub 2015 Jun 30. Nat Rev Clin Oncol. 2015. PMID: 26122185 Free PMC article. Review.
References
- Tsatmali M, Walcott EC, Crossin KL. Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res. 2005;1040:137–50. - PubMed
- Ito K, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. - PubMed
- Ito K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–51. - PubMed
- Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA100225/CA/NCI NIH HHS/United States
- R01 CA100225-05/CA/NCI NIH HHS/United States
- U54 CA126524/CA/NCI NIH HHS/United States
- U54 CA126524-04/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases