A psychological and neuroanatomical model of obsessive-compulsive disorder - PubMed (original) (raw)
Review
A psychological and neuroanatomical model of obsessive-compulsive disorder
Edward D Huey et al. J Neuropsychiatry Clin Neurosci. 2008 Fall.
Abstract
Imaging, surgical, and lesion studies suggest that the prefrontal cortex (orbitofrontal and anterior cingulate cortexes), basal ganglia, and thalamus are involved in the pathogenesis of obsessive-compulsive disorder (OCD). On the basis of these findings several models of OCD have been developed, but have had difficulty fully integrating the psychological and neuroanatomical findings of OCD. Recent research in the field of cognitive neuroscience on the normal function of these brain areas demonstrates the role of the orbitofrontal cortex in reward, the anterior cingulate cortex in error detection, the basal ganglia in affecting the threshold for activation of motor and behavioral programs, and the prefrontal cortex in storing memories of behavioral sequences (called "structured event complexes" or SECs). The authors propose that the initiation of these SECs can be accompanied by anxiety that is relieved with completion of the SEC, and that a deficit in this process could be responsible for many of the symptoms of OCD. Specifically, the anxiety can form the basis of an obsession, and a compulsion can be an attempt to receive relief from the anxiety by repeating parts of, or an entire, SEC. The authors discuss empiric support for, and specific experimental predictions of, this model. The authors believe that this model explains the specific symptoms, and integrates the psychology and neuroanatomy of OCD better than previous models.
Figures
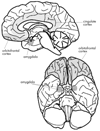
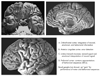
FIGURE 1. The Key Brain Structures Implicated in Reward and Emotion
The position of the amygdala, orbitofrontal cortex and cingulate cortex are shown on a midsagittal view (top), and on a ventral view (bottom) of the human brain. Reproduced with permission from Luxenberg et al. 1988 (114)
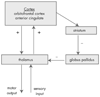
FIGURE 2. Model of Interaction of Basal Ganglia with Other Brain Structures
GPi = internal segment of globus pallidus; GPe = external segment of globus pallidus; SNc = substantia nigra pars compacta; SNr = substantia nigra pars reticulata; VTA = ventral tegmental area; ABL = basolateral amygdala. Reproduced with permission from Frank et al. 2006 (54)
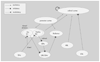
FIGURE 3. Reward Values Associated with Active SECs at a Given Time
This figure shows a hypothesized schematic representation of the changes in a few active motivational/reward states. The overall reward state at a given time of an animal will be the summation of the component reward states, and the emotional “flavor” of the reward state is provided through interactions with limbic structures. SECs = structured event complexes
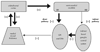
FIGURE 4. The Standard Model of OCD
OCD = obsessive-compulsive disorder. Excitatory connections are labeled +; inhibitory connections are labeled −. Reproduced with Permission from Rauch et al. 2006 (94)
FIGURE 5. A Neuroanatomical Model That Incorporates Direct and Indirect Striatal Pathways
GPi = globus pallidus interna; SNr = substantia nigra pars reticulata In OCD, the direct pathway is strongly activated in relation to the indirect pathway resulting in OFC-subcortical hyperactivity. Large arrows represent inputs that are strengthened in patients with OCD. Reproduced with permission from Stein 2006 (96)
FIGURE 6. Schematic Representation of the SEC/OCD Model
Brain areas are listed with summary of function. In healthy people, initiation of an SEC can generate motivational anxiety. Completion of such an SEC results in a reward signal and a reduction in anxiety. People with OCD do not receive the full reward signal and reduction of anxiety upon completion of an SEC, giving them the sensation of leaving a task undone, which they attempt to remove by repeatedly performing an SEC or segments of an SEC. Symptoms of OCD can be acquired by damage to the basal ganglia, OFC, or ACC. OCD = obsessive-compulsive disorder; SEC = structured event complex; OFC = orbitofrontal cortex; ACC = anterior cingulate cortex;
Similar articles
- [Present contribution of neurosciences to a new clinical reading of obsessive-compulsive disorder].
Aouizerate B, Rotgé JY, Bioulac B, Tignol J. Aouizerate B, et al. Encephale. 2007 Mar-Apr;33(2):203-10. doi: 10.1016/s0013-7006(07)91551-1. Encephale. 2007. PMID: 17675916 Review. French. - Meta-analysis of brain volume changes in obsessive-compulsive disorder.
Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M, Burbaud P, Aouizerate B. Rotge JY, et al. Biol Psychiatry. 2009 Jan 1;65(1):75-83. doi: 10.1016/j.biopsych.2008.06.019. Epub 2008 Aug 21. Biol Psychiatry. 2009. PMID: 18718575 - Role of the dorsal anterior cingulate cortex in obsessive-compulsive disorder: converging evidence from cognitive neuroscience and psychiatric neurosurgery.
McGovern RA, Sheth SA. McGovern RA, et al. J Neurosurg. 2017 Jan;126(1):132-147. doi: 10.3171/2016.1.JNS15601. Epub 2016 Apr 1. J Neurosurg. 2017. PMID: 27035167 - Morphometric brain characterization of refractory obsessive-compulsive disorder: diffeomorphic anatomic registration using exponentiated Lie algebra.
Tang W, Li B, Huang X, Jiang X, Li F, Wang L, Chen T, Wang J, Gong Q, Yang Y. Tang W, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2013 Oct 1;46:126-31. doi: 10.1016/j.pnpbp.2013.07.011. Epub 2013 Jul 19. Prog Neuropsychopharmacol Biol Psychiatry. 2013. PMID: 23876787 - Altered anatomical connections of associative and limbic cortico-basal-ganglia circuits in obsessive-compulsive disorder.
Haynes WIA, Clair AH, Fernandez-Vidal S, Gholipour B, Morgiève M, Mallet L. Haynes WIA, et al. Eur Psychiatry. 2018 Jun;51:1-8. doi: 10.1016/j.eurpsy.2018.01.005. Epub 2018 Mar 4. Eur Psychiatry. 2018. PMID: 29514116
Cited by
- Subcortical shape in pediatric and adult obsessive-compulsive disorder.
Wang Z, Fontaine M, Cyr M, Rynn MA, Simpson HB, Marsh R, Pagliaccio D. Wang Z, et al. Depress Anxiety. 2022 Jun;39(6):504-514. doi: 10.1002/da.23261. Epub 2022 Apr 29. Depress Anxiety. 2022. PMID: 35485920 Free PMC article. - Separate mechanisms for development and performance of compulsive checking in the quinpirole sensitization rat model of obsessive-compulsive disorder (OCD).
Tucci MC, Dvorkin-Gheva A, Sharma R, Taji L, Cheon P, Peel J, Kirk A, Szechtman H. Tucci MC, et al. Psychopharmacology (Berl). 2014 Sep;231(18):3707-18. doi: 10.1007/s00213-014-3505-6. Epub 2014 Feb 28. Psychopharmacology (Berl). 2014. PMID: 24682503 - N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action.
Dean O, Giorlando F, Berk M. Dean O, et al. J Psychiatry Neurosci. 2011 Mar;36(2):78-86. doi: 10.1503/jpn.100057. J Psychiatry Neurosci. 2011. PMID: 21118657 Free PMC article. Review. - The implication of neuroactive steroids in Tourette's syndrome pathogenesis: A role for 5α-reductase?
Bortolato M, Frau R, Godar SC, Mosher LJ, Paba S, Marrosu F, Devoto P. Bortolato M, et al. J Neuroendocrinol. 2013 Nov;25(11):1196-208. doi: 10.1111/jne.12066. J Neuroendocrinol. 2013. PMID: 23795653 Free PMC article. Review. - Neurocognitive deficits in obsessive-compulsive disorder: A selective review.
Suhas S, Rao NP. Suhas S, et al. Indian J Psychiatry. 2019 Jan;61(Suppl 1):S30-S36. doi: 10.4103/psychiatry.IndianJPsychiatry_517_18. Indian J Psychiatry. 2019. PMID: 30745674 Free PMC article. Review.
References
- Janet P. Les Obsessions et la Psychasthenie [Obsessions and Psychasthenia] 2nd ed. Paris: Alcan; 1903.
- Karno M, Golding JM, Sorenson SB, et al. The epidemiology of obsessive-compulsive disorder in five US communities. Arch Gen Psychiatry. 1988;45:1094–1099. - PubMed
- Stein DJ. Obsessive-compulsive disorder. Lancet. 2002;360:397–405. - PubMed
- Foa EB, Kozak MJ, Goodman WK, et al. DSM-IV field trial: obsessive-compulsive disorder. Am J Psychiatry. 1995;152:90–96. - PubMed
- Friedlander L, Desrocher M. Neuroimaging studies of obsessive-compulsive disorder in adults and children. Clin Psychol Rev. 2006;26:32–49. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical