The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response - PubMed (original) (raw)
The G protein-coupled receptor FSHR-1 is required for the Caenorhabditis elegans innate immune response
Jennifer R Powell et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Innate immunity is an ancient defense system used by both vertebrates and invertebrates. Previously characterized innate immune responses in plants and animals are triggered by detection of pathogens using specific receptors, which typically use a leucine-rich repeat (LRR) domain to bind molecular patterns associated with infection. The nematode Caenorhabditis elegans uses defense pathways conserved with vertebrates; however, the mechanism by which C. elegans detects pathogens is unknown. We screened all LRR-containing transmembrane receptors in C. elegans and identified the G protein-coupled receptor FSHR-1 as an important component of the C. elegans immune response to Gram-negative and Gram-positive bacterial pathogens. FSHR-1 acts in the C. elegans intestine, the primary site of exposure to ingested pathogens. FSHR-1 signals in parallel to the known p38 MAPK pathway but converges to regulate the transcriptional induction of an overlapping but nonidentical set of antimicrobial effectors. FSHR-1 may act generally to boost the nematode immune response, or it may function as a pathogen receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
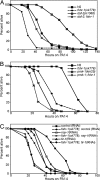
Fig. 1.
FSHR-1 is required for innate immunity. (A and B) fshr-1 (RNAi) or fshr-1(ok778) mutant worms are sensitive to killing by pathogenic P. aeruginosa PA14 relative to wild-type control worms. (C) fshr-1(ok778)/nDf31 heterozygotes are just as sensitive to PA14 as fshr-1(ok778) homozygotes. Parent nDf31/+ worms (actual genotype: nDf31/nT1[qIs51]) and sibling fshr-1(ok778)/+ worms (actual genotype: fshr-1(ok778)/nT1[qIs51]) are not sensitive to PA14. (D–F) fshr-1(ok778) mutants are sensitive to killing by pathogenic S. aureus and E. faecalis but not to E. coli OP50.
Fig. 2.
FSHR-1 acts in parallel to DAF-2 and the p38 MAPK pathway. (A) daf-2 and fshr-1 single and double mutants were raised at 15 °C and then shifted to the restrictive temperature of 25 °C 4 h before exposure to PA14. (B and C) Loss of components of the p38 MAPK pathway, either by genetic mutation (pmk-1) or RNAi (tir-1 and nsy-1), enhances the pathogen sensitivity of fshr-1(ok778) null mutants. Experiments with pmk-1 and fshr-1 single and double mutants were repeated 5 times, and in all cases the double mutants were significantly more sensitive than either single mutant (P < 0.01 4 out of 5 times; P < 0.05 1 out of 5 times).
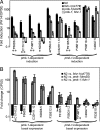
Fig. 3.
FSHR-1 regulates PA14-response genes. (A and B) qRT-PCR was used to analyze the relative transcription of PA14-response genes in wild-type and mutant worms fed OP50 or PA14. Error bars represent SEM for 3 independent biologic replicates. (A) Fold induction was calculated as the ratio of normalized expression on PA14 divided by expression on OP50. *Genes with greater than 5-fold reduction in their induction in fshr-1(ok778) mutant worms relative to wild-type worms with P < 0.01. **Genes with greater than 10-fold reduction in their induction in fshr-1(ok778) mutants relative to wild-type with P < 0.01. (B) Fold change in basal expression was calculated as the ratio of wild-type expression to mutant expression in worms fed OP50. Troemel et al. (15) reported that F01D5.5 was regulated basally by PMK-1, but in our experiments the difference did not reach statistical significance. *Genes with greater than 5-fold lower basal expression in wild-type worms relative to fshr-1(ok778) mutants. **Genes with greater than 10-fold lower basal expression in wild-type worms relative to fshr-1(ok778) mutants.
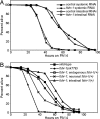
Fig. 4.
FSHR-1 expression in the intestine is necessary and sufficient for pathogen resistance. (A) Intestinal-specific RNAi of fshr-1 causes worms to be as sensitive to PA14 as systemic RNAi of fshr-1. (B) Wild-type FSHR-1 expressed from endogenous or intestinal promoters fully rescues the fshr-1(ok778) mutant phenotype. FSHR-1 expressed from a neuronal promoter only partially rescues the mutant phenotype.
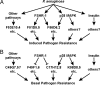
Fig. 5.
A model for a C. elegans innate immune network. (A and B) This model shows the integration of signaling from 3 known innate immune pathways. (A) Each pathway regulates the induction of a set of effectors in response to P. aeruginosa. The FSHR-1 and p38 MAPK pathways regulate partially overlapping sets of pathogen-response genes. Specific examples of target genes mentioned in this work are listed for each category. (B) These signaling pathways also regulate the basal expression in the absence of pathogen of several genes. Examples mentioned here are listed for each category.
Similar articles
- System wide analysis of the evolution of innate immunity in the nematode model species Caenorhabditis elegans and Pristionchus pacificus.
Sinha A, Rae R, Iatsenko I, Sommer RJ. Sinha A, et al. PLoS One. 2012;7(9):e44255. doi: 10.1371/journal.pone.0044255. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23028509 Free PMC article. - IGLR-2, a Leucine-Rich Repeat Domain Containing Protein, Is Required for the Host Defense in Caenorhabditis elegans.
Kuo CJ, Hsu YC, Wang ST, Liou BY, Lim SB, Chen YW, Chen CS. Kuo CJ, et al. Front Immunol. 2020 Nov 30;11:561337. doi: 10.3389/fimmu.2020.561337. eCollection 2020. Front Immunol. 2020. PMID: 33329523 Free PMC article. - Aberrant Activation of p38 MAP Kinase-Dependent Innate Immune Responses Is Toxic to Caenorhabditis elegans.
Cheesman HK, Feinbaum RL, Thekkiniath J, Dowen RH, Conery AL, Pukkila-Worley R. Cheesman HK, et al. G3 (Bethesda). 2016 Jan 27;6(3):541-9. doi: 10.1534/g3.115.025650. G3 (Bethesda). 2016. PMID: 26818074 Free PMC article. - Specificity of the innate immune system and diversity of C-type lectin domain (CTLD) proteins in the nematode Caenorhabditis elegans.
Schulenburg H, Hoeppner MP, Weiner J 3rd, Bornberg-Bauer E. Schulenburg H, et al. Immunobiology. 2008;213(3-4):237-50. doi: 10.1016/j.imbio.2007.12.004. Epub 2008 Feb 11. Immunobiology. 2008. PMID: 18406370 Review. - The worm has turned--microbial virulence modeled in Caenorhabditis elegans.
Sifri CD, Begun J, Ausubel FM. Sifri CD, et al. Trends Microbiol. 2005 Mar;13(3):119-27. doi: 10.1016/j.tim.2005.01.003. Trends Microbiol. 2005. PMID: 15737730 Review.
Cited by
- NPR-9 regulates the innate immune response in Caenorhabditis elegans by antagonizing the activity of AIB interneurons.
Yu Y, Zhi L, Wu Q, Jing L, Wang D. Yu Y, et al. Cell Mol Immunol. 2018 Jan;15(1):27-37. doi: 10.1038/cmi.2016.8. Epub 2016 May 2. Cell Mol Immunol. 2018. PMID: 27133473 Free PMC article. - Spatial transcriptomics reveals antiparasitic targets associated with essential behaviors in the human parasite Brugia malayi.
Airs PM, Vaccaro K, Gallo KJ, Dinguirard N, Heimark ZW, Wheeler NJ, He J, Weiss KR, Schroeder NE, Huisken J, Zamanian M. Airs PM, et al. PLoS Pathog. 2022 Apr 7;18(4):e1010399. doi: 10.1371/journal.ppat.1010399. eCollection 2022 Apr. PLoS Pathog. 2022. PMID: 35390105 Free PMC article. - Genomic and Experimental Evidence Suggests that Verrucomicrobium spinosum Interacts with Eukaryotes.
Sait M, Kamneva OK, Fay DS, Kirienko NV, Polek J, Shirasu-Hiza MM, Ward NL. Sait M, et al. Front Microbiol. 2011 Oct 18;2:211. doi: 10.3389/fmicb.2011.00211. eCollection 2011. Front Microbiol. 2011. PMID: 22022322 Free PMC article. - G protein-coupled receptors: The choreographers of innate immunity in Caenorhabditis elegans.
Venkatesh SR, Singh V. Venkatesh SR, et al. PLoS Pathog. 2021 Jan 21;17(1):e1009151. doi: 10.1371/journal.ppat.1009151. eCollection 2021 Jan. PLoS Pathog. 2021. PMID: 33476324 Free PMC article. Review. No abstract available. - Immune defense mechanisms in the Caenorhabditis elegans intestinal epithelium.
Pukkila-Worley R, Ausubel FM. Pukkila-Worley R, et al. Curr Opin Immunol. 2012 Feb;24(1):3-9. doi: 10.1016/j.coi.2011.10.004. Epub 2012 Jan 9. Curr Opin Immunol. 2012. PMID: 22236697 Free PMC article. Review.
References
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. - PubMed
- Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. - PubMed
- Schulenburg H, Kurz CL, Ewbank JJ. Evolution of the innate immune system: The worm perspective. Immunol Rev. 2004;198:36–58. - PubMed
- Sifri CD, Begun J, Ausubel FM. The worm has turned—Microbial virulence modeled in Caenorhabditis elegans. Trends Microbiol. 2005;13:119–127. - PubMed
- Kim DH, Ausubel FM. Evolutionary perspectives on innate immunity from the study of Caenorhabditis elegans. Curr Opin Immunol. 2005;17:4–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 AI068376/AI/NIAID NIH HHS/United States
- P01 AI044220/AI/NIAID NIH HHS/United States
- R01 AI064332/AI/NIAID NIH HHS/United States
- R01 GM084477/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases