Post-translational inhibition of IP-10 secretion in IEC by probiotic bacteria: impact on chronic inflammation - PubMed (original) (raw)
Post-translational inhibition of IP-10 secretion in IEC by probiotic bacteria: impact on chronic inflammation
Gabriele Hoermannsperger et al. PLoS One. 2009.
Erratum in
- PLoS One. 2009;4(6). doi: 10.1371/annotation/583d95a8-c18c-4c66-92be-1b1505802d86. Hörmannsperger, Gabriele [corrected to Hoermannsperger, Gabriele]
Abstract
Background: Clinical and experimental studies suggest that the probiotic mixture VSL#3 has protective activities in the context of inflammatory bowel disease (IBD). The aim of the study was to reveal bacterial strain-specific molecular mechanisms underlying the anti-inflammatory potential of VSL#3 in intestinal epithelial cells (IEC).
Methodology/principal findings: VSL#3 inhibited TNF-induced secretion of the T-cell chemokine interferon-inducible protein (IP-10) in Mode-K cells. Lactobacillus casei (L. casei) cell surface proteins were identified as active anti-inflammatory components of VSL#3. Interestingly, L. casei failed to block TNF-induced IP-10 promoter activity or IP-10 gene transcription at the mRNA expression level but completely inhibited IP-10 protein secretion as well as IP-10-mediated T-cell transmigration. Kinetic studies, pulse-chase experiments and the use of a pharmacological inhibitor for the export machinery (brefeldin A) showed that L. casei did not impair initial IP-10 production but decreased intracellular IP-10 protein stability as a result of blocked IP-10 secretion. Although L. casei induced IP-10 ubiquitination, the inhibition of proteasomal or lysosomal degradation did not prevent the loss of intracellular IP-10. Most important for the mechanistic understanding, the inhibition of vesicular trafficking by 3-methyladenine (3-MA) inhibited IP-10 but not IL-6 expression, mimicking the inhibitory effects of L. casei. These findings suggest that L. casei impairs vesicular pathways important for the secretion of IP-10, followed by subsequent degradation of the proinflammatory chemokine. Feeding studies in TNF(DeltaARE) and IL-10(-/-) mice revealed a compartimentalized protection of VSL#3 on the development of cecal but not on ileal or colonic inflammation. Consistent with reduced tissue pathology in IL-10(-/-) mice, IP-10 protein expression was reduced in primary epithelial cells.
Conclusions/significance: We demonstrate segment specific effects of probiotic intervention that correlate with reduced IP-10 protein expression in the native epithelium. Furthermore, we revealed post-translational degradation of IP-10 protein in IEC to be the molecular mechanism underlying the anti-inflammatory effect.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
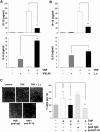
Figure 1. L. casei selectively inhibits TNF-induced secretion of chemotactically functional IP-10.
Mode-K cells were stimulated for 24 h with (A) VSL#3 (moi 20) or (B) L. casei (moi 20) with or without TNF (10 ng/ml) activation for 24 h. The concentration of IP-10 and IL-6 in cell culture supernatants was measured by ELISA. Bars represent mean values (+/− SD) of triplicate samples. (C) 100 µl DMEM or cell culture supernatant from a 24 h stimulation experiment with Mode-K cells (control, TNF (10 ng/ml), TNF+L. casei (moi 20)) were added to 500 µl of transmigration medium. The amount of transmigrated cells per region of interest was determined by axiovision cell counting. Pictures show representative sections of transmigrated cells of three independent experiments and bars represent mean values (+/− SD) of triplicate samples.
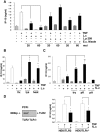
Figure 2. The inhibition of TNF-induced IP-10 secretion is mediated by a surface protein of L. casei.
(A) Mode-K cells were stimulated for 24 h with L. casei, Lactobacillus plantarum 299v or E. coli Nissle 1917 (moi as indicated) with or without TNF (10 ng/ml) activation for 24 h. The concentration of IP-10 and IL-6 in cell culture supernatants was measured by ELISA. Bars represent mean values (+/− SD) of triplicate samples. (B) Mode-K cells were stimulated with L. casei (moi 20) killed by formaldehyde fixation (fix), lysozyme (lys)-digestion or heat (heat)-treatment with or without TNF (10 ng/ml) activation for 24 h. (C) L. casei was treated with trypsin (try), proteinase K (pK) or phospholipase A (pA) followed by formaldehyde-fixation (fL.c). (D) TLR2 deficient as well as TLR2 expressing HEK293 cells (absence/presence of TLR2 in the cells was analysed by real-time PCR) were stimulated for 24 h with TNF (10 ng/ml) and L. casei (moi 20). IP-10 concentration was measured in the culture supernatants by ELISA. The bars represent mean values (+/− SD) of triplicate samples.
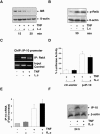
Figure 3. _L. casei_-induced inhibition of IP-10 secretion is mediated by a post-transcriptional mechanism
(A) Mode-K cells were stimulated with TNF (10 ng/ml) and L. casei (moi 20) for 15 and 30 min and TNF-induced proteasomal degradation of IκB and (B) TNF-induced activation of RelA was analyzed by Western Blot in two independent experiments. (C) TNF-induced recruitment of NFκB RelA to the IP-10 promoter was analyzed in a ChIP experiment using an anti-RelA antibody after pre-incubation of Mode-K cells with L. casei (moi 20) (1h) and subsequent stimulation with TNF (10 ng/ml) for 2 h. (D) Control (ctr-vector)- and IP-10 reporter (p-IP-10)-plasmid transfected Mode-K cells were stimulated with TNF (10 ng/ml) with or without L. casei (moi 20) and intracellular luciferase activity was measured by a luminescence assay after 24 h. (E) Mode-K cells were stimulated with TNF (10 ng/ml) and/or L. casei (Moi 20) for 6 h followed by mRNA isolation, RT-PCR and qPCR. (F) Mode-K cells were stimulated with TNF (10 ng/ml) alone and together with L. casei (moi 20) for 24 h. Intracellular accumulation of IP-10 was analyzed by Western blot and the shown figure is representative for more than three independent experiments.
Figure 4. The inhibition of IP-10 secretion is independent of the signal-specific activation of IEC.
(A) Mode-K cells were stimulated for 24 h with IFNγ (100 ng/ml) and L. casei (moi 20). Cell culture supernatants were analyzed by ELISA and (B) intracellular IP-10 protein levels by Western blot. (C) Mode-K cells were transfected with an IP-10 overexpression (pIP-10-DsRed)- or a ctr-DsRed-plasmid (transfection control) for 6 h and were then treated with L. casei for 24 h. IP-10 levels in the supernatants were determined by ELISA and intracellular DsRed-tagged IP-10 (IP-10-DsRed) or DsRed levels (transfection control) were determined by Western blot. Bars in A/C represent mean values (+/− SD) of triplicate samples and all figures are representative for three independent experiments.
Figure 5. Degradation of IP-10 protein is the result of an IP-10 specific secretional blockade.
(A) Mode-K cells were stimulated for 1, 3 or 24 h with TNF (10 ng/ml) alone or together with _L. case_i (moi 20) and intracellular as well as secreted levels of IP-10 were analyzed by Western Blot or ELISA analysis. Bars in A represent mean values (+/− SD) of triplicate samples. The shown figure is representative for three independent experiments. (B) Mode-K cells were stimulated with TNF (10 ng/ml) during a 3h pulse period (S35-labelled cysteine/methionine, 25 µCi) followed by a 3 h chase period in DMEM supplemented with L. casei (moi 20) or not. Subsequent immunoprecipitation for IP-10 was followed by protein separation on a 15% SDS gel. Intracellular levels of S35-labelled IP-10 were then made visible by a Phosphoimager plate. The shown figure is representative for two independent experiments. (C) Mode-K cells were stimulated with TNF (10 ng/ml) alone or together with L. casei in the presence or absence of brefeldin A (0,5 µM). The amount of intracellular IP-10 after 6 h of stimulation was analyzed by Western blot. (D) Mode-K cells were stimulated for 6 h with TNF (10 ng/ml) alone or with L. casei (moi 20) before lysis and subsequent immunoprecipitation using an anti-IP-10 antibody was performed. Western blot was performed to investigate the presence of IP-10 and ubiquitine in the precipitated fractions (single experiment). (E) Mode-K cells were stimulated with IFNγ (100 ng/ml) or TNF (10 ng/ml) alone or together with L. casei (moi 20) in the presence of lactacystin or NH4Cl for 24h and intracellular IP-10 was analyzed by Western blot. The shown figures are representative for three independent experiments.
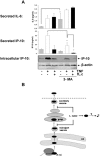
Figure 6. Blockade of 3-MA-dependent vesicular transport by L. casei might induce the degradation of IP-10.
(A) Mode-K cells were stimulated with TNF (10 ng/ml) alone or together with L. casei (moi 20) in the presence or absence of 3-MA for 24 h and the amount of IP-10 in the cell culture supernatant as well as intracellularly was analyzed by ELISA and Western blot. Figure A is representative for three independent experiments and bars represent mean values (+/− SD) of triplicate samples. (B) The scheme illustrates our hypothesis that L. casei might target 3-MA dependent vesicular transport of IP-10 resulting in a secretional blockade and subsequent degradation of the chemokine.
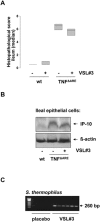
Figure 7. VSL#3 does not reduce experimental ileitis in TNFΔARE mice.
TNFΔARE (n = 6) and wt mice (n = 5) were fed VSL#3 (1,3×109 cfu/mouse/day) or placebo for 15 weeks. Mice were sacrificed at 18 weeks of age and histopathological scoring was performed by blindly assessing the inflammation between 0 (not inflamed) and 12 (highly inflamed). Figure A shows the median histopathologic score of TNFΔARE mice in the study. (B) Ileal IEC were isolated and pooled proteins (50 µg) of all mice in a group were analyzed for IP-10 expression by Western blot. (C) The presence of VSL#3 derived S. thermophilus (S.t) in the gut of the TNFΔARE mice was examined by bacteria-specific PCR analysis.
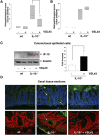
Figure 8. Intestinal segment specific effects of VSL#3 on experimental inflammation correlate with IP-10 expression in IEC.
IL-10−/− mice were fed VSL#3 (1,3×109 cfu/mouse/day) (n = 14) or placebo (n = 12) analogous to wt mice (n = 9) for 21 weeks. Mice were sacrificed at 24 weeks of age and histopathological scoring was performed by blindly assessing the inflammation between 0 (not inflamed) and 12 (highly inflamed). Figure A and B show the median histopathologic score of the cecum and colon of IL-10−/− mice in the study. (C) Cecal and colonic IEC were isolated together and proteins (50 µg) as well as mRNA of all mice in a group were analyzed for IP-10 expression by Western blot or RT-PCR (D) Immunohistochemical staining of IP-10 in cecal tissue sections was performed. The upper row shows representative pictures (three mice per group were analysed) of stained tissue sections (IP-10 (green), DAPI (blue)) and the lower row shows representative false colour confocal images of the stained tissue sections ((IP-10) green, DAPI (red)). Arrows indicate regions that show elevated IP-10 expression.
Similar articles
- Posttranslational inhibition of proinflammatory chemokine secretion in intestinal epithelial cells: implications for specific IBD indications.
Hörmannsperger G, Clavel T, Hoffmann M, Reiff C, Kelly D, Loh G, Blaut M, Hölzlwimmer G, Haller D. Hörmannsperger G, et al. J Clin Gastroenterol. 2010 Sep;44 Suppl 1:S10-5. doi: 10.1097/MCG.0b013e3181e102c1. J Clin Gastroenterol. 2010. PMID: 20562631 - Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines.
von Schillde MA, Hörmannsperger G, Weiher M, Alpert CA, Hahne H, Bäuerl C, van Huynegem K, Steidler L, Hrncir T, Pérez-Martínez G, Kuster B, Haller D. von Schillde MA, et al. Cell Host Microbe. 2012 Apr 19;11(4):387-96. doi: 10.1016/j.chom.2012.02.006. Cell Host Microbe. 2012. PMID: 22520466 - Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment.
Zakostelska Z, Kverka M, Klimesova K, Rossmann P, Mrazek J, Kopecny J, Hornova M, Srutkova D, Hudcovic T, Ridl J, Tlaskalova-Hogenova H. Zakostelska Z, et al. PLoS One. 2011;6(11):e27961. doi: 10.1371/journal.pone.0027961. Epub 2011 Nov 22. PLoS One. 2011. PMID: 22132181 Free PMC article. - Lactocepin as a protective microbial structure in the context of IBD.
Hörmannsperger G, von Schillde MA, Haller D. Hörmannsperger G, et al. Gut Microbes. 2013 Mar-Apr;4(2):152-7. doi: 10.4161/gmic.23444. Epub 2013 Jan 18. Gut Microbes. 2013. PMID: 23333860 Free PMC article. Review.
Cited by
- Occurrence and Dynamism of Lactic Acid Bacteria in Distinct Ecological Niches: A Multifaceted Functional Health Perspective.
George F, Daniel C, Thomas M, Singer E, Guilbaud A, Tessier FJ, Revol-Junelles AM, Borges F, Foligné B. George F, et al. Front Microbiol. 2018 Nov 27;9:2899. doi: 10.3389/fmicb.2018.02899. eCollection 2018. Front Microbiol. 2018. PMID: 30538693 Free PMC article. Review. - Temporal Changes in Vaginal Microbiota and Genital Tract Cytokines Among South African Women Treated for Bacterial Vaginosis.
Mtshali A, San JE, Osman F, Garrett N, Balle C, Giandhari J, Onywera H, Mngomezulu K, Mzobe G, de Oliveira T, Rompalo A, Mindel A, Abdool Karim SS, Ravel J, Passmore JS, Abdool Karim Q, Jaspan HB, Liebenberg LJP, Ngcapu S. Mtshali A, et al. Front Immunol. 2021 Sep 14;12:730986. doi: 10.3389/fimmu.2021.730986. eCollection 2021. Front Immunol. 2021. PMID: 34594336 Free PMC article. - Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration.
Ruan W, Engevik MA, Spinler JK, Versalovic J. Ruan W, et al. Dig Dis Sci. 2020 Mar;65(3):695-705. doi: 10.1007/s10620-020-06118-4. Dig Dis Sci. 2020. PMID: 32067143 Review. - Cross-Sectional Analysis of Selected Genital Tract Immunological Markers and Molecular Vaginal Microbiota in Sub-Saharan African Women, with Relevance to HIV Risk and Prevention.
Kyongo JK, Crucitti T, Menten J, Hardy L, Cools P, Michiels J, Delany-Moretlwe S, Mwaura M, Ndayisaba G, Joseph S, Fichorova R, van de Wijgert J, Vanham G, Ariën KK, Jespers V. Kyongo JK, et al. Clin Vaccine Immunol. 2015 May;22(5):526-38. doi: 10.1128/CVI.00762-14. Epub 2015 Mar 11. Clin Vaccine Immunol. 2015. PMID: 25761460 Free PMC article. - A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa.
Jespers V, Kyongo J, Joseph S, Hardy L, Cools P, Crucitti T, Mwaura M, Ndayisaba G, Delany-Moretlwe S, Buyze J, Vanham G, van de Wijgert JHHM. Jespers V, et al. Sci Rep. 2017 Sep 20;7(1):11974. doi: 10.1038/s41598-017-12198-6. Sci Rep. 2017. PMID: 28931859 Free PMC article.
References
- Loftus E. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gasteroenterology. 2004;126:1504–1517. - PubMed
- Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. - PubMed
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. - PubMed
- Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202–1209. - PubMed
- Gionchetti P, Rizzello F, Morselli C, Poggioli G, Tambasco R, et al. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum. 2007;50:2075–2082. discussion 2082–2074. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources