Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill - PubMed (original) (raw)
Comparative Study
Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill
Henry H Yin et al. Nat Neurosci. 2009 Mar.
Abstract
The learning of new skills is characterized by an initial phase of rapid improvement in performance and a phase of more gradual improvements as skills are automatized and performance asymptotes. Using in vivo striatal recordings, we observed region-specific changes in neural activity during the different phases of skill learning, with the associative or dorsomedial striatum being preferentially engaged early in training and the sensorimotor or dorsolateral striatum being engaged later in training. Ex vivo recordings from medium spiny striatal neurons in brain slices of trained mice revealed that the changes observed in vivo corresponded to regional- and training-specific changes in excitatory synaptic transmission in the striatum. Furthermore, the potentiation of glutamatergic transmission observed in dorsolateral striatum after extensive training was preferentially expressed in striatopallidal neurons, rather than striatonigral neurons. These findings demonstrate that region- and pathway-specific plasticity sculpts the circuits involved in the performance of the skill as it becomes automatized.
Figures
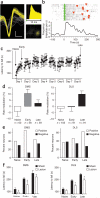
Figure 1
In vivo recordings of neuronal activity in the DMS and DLS striatal regions during different phases of skill learning. (a) Example of a single-unit waveform (vertical bar 20 μV; horizontal 200 μs), interspike interval and cluster separation from noise on the basis of principal component analyses. (b) Example of a putative medium spiny neuron that increased firing rate (impulses per second) when the animal was running on the rotarod versus baseline (intertrial period). (c) Latency to fall off the rotarod throughout training for the implanted animals. (d) Average modulation of firing rate during running versus baseline in the DMS and DLS during the different phases of skill learning. The number of neurons recorded in the six animals is shown for each condition. (e) Percentage of task-related neurons that increased versus decreased firing rate when the animal was running in the rotarod compared with baseline in DMS and DLS during the different phases of skill learning. (f) Effects of excitotoxic lesions in DMS and DLS on the different phases of skill learning. Error bars indicate s.e.m.
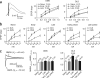
Figure 2
Ex vivo striatal field potential recordings in the DMS and DLS during different phases of skill learning. (a) Performance of the animals on the accelerating rotarod for the early and late groups of ex vivo electrophysiology experiments. (b) Placement of the stimulating and recording electrodes for the ex vivo experiments. (c) Input-output function of striatal population spike amplitude versus afferent stimulation strength in the DMS and DLS. (d) Half-maximal evoked striatal population spike amplitude in the DMS and DLS. (e) Number of LTD tetani needed to reach saturation in the DMS and DLS. (f) LTD saturation in the DMS and DLS. The number of animals used for every condition is indicated. Representative traces are shown on the right. Error bars indicate s.e.m.
Figure 3
Ex vivo striatal whole cell recordings in the DMS and DLS during the different phases of skill learning. (a) Input-output function of EPSP slope versus stimulation strength. Representative traces are presented on the left. (b) The same measure is presented as in a, directly comparing synaptic strength in the DMS and DLS during the different phases of skill learning and in yoked controls. (c) NMDA/AMPA ratio in DMS and DLS during the different phases of skill learning. Representative traces are shown on the left. The number of neurons and number of animals (between brackets) are shown for each condition. Error bars indicate s.e.m.
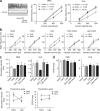
Figure 4
Training results in region-specific changes in excitability and in threshold to spike in the DLS and the DMS. (a) Changes in excitability in DMS and DLS during the different phases of skill learning. Representative traces are shown on the left. The number of neurons and number of animals (between brackets) are shown for each condition. (b) The same measure is presented as in a, directly comparing excitability in the DMS and DLS during the different phases of skill learning. (c) Threshold afferent stimulation strength for spiking. (d) Threshold EPSP slope for spiking. (e) The afferent stimulation strength, but not the EPSP slope, necessary to produce spike changes dynamically in DMS and DLS across the different phases of skill learning. Error bars indicate s.e.m.
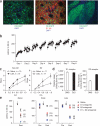
Figure 5
Extensive training results in pathway-specific plasticity. (a) GFP in D2-EGFP mice was not expressed in all medium spiny neurons (left, EGFP expression in green and nuclear staining with DAPI in blue). D1 receptor expression did not colocalize with GFP expression in D2-EGFP mice (middle, EGFP expression in green, D1 antibody staining in red and DAPI in blue). Visualization of striatal projections to globus pallidus revealed that GFP labeled cells were mainly striatopallidal neurons in D2-EGFP mice (right, EGFP expression in green and DAPI in blue). (b) Performance of D2-EGFP mice on the rotarod. (c) Whole cell recordings of GFP-positive cells and GFP-negative cells in D2-EGFP mice revealed long-lasting potentiation of glutamatergic transmission in the dorsolateral striatum, preferentially in striatopallidal neurons. (d) Binding studies in C57Bl6/J revealed that D2 membrane expression was much higher in DLS than in DMS, whereas D1 expression was more similar between DMS and DLS. (e) Acute blockade of D1, D2, or D1 and D2 type dopamine receptors in naive animals and during the early and late phase of training revealed that performance in the rotarod became independent of D1-type dopamine receptor activation with extensive training, but was dependent on D2-type dopamine receptor activation. Error bars indicate s.e.m.
Similar articles
- Temporal dynamics of Arc/Arg3.1 expression in the dorsal striatum during acquisition and consolidation of a motor skill in mice.
Gong WK, Ni J, Yu LF, Wang L, Huang ZL. Gong WK, et al. Neurobiol Learn Mem. 2020 Feb;168:107156. doi: 10.1016/j.nlm.2019.107156. Epub 2020 Jan 2. Neurobiol Learn Mem. 2020. PMID: 31904548 - Multimodal Plasticity in Dorsal Striatum While Learning a Lateralized Navigation Task.
Hawes SL, Evans RC, Unruh BA, Benkert EE, Gillani F, Dumas TC, Blackwell KT. Hawes SL, et al. J Neurosci. 2015 Jul 22;35(29):10535-49. doi: 10.1523/JNEUROSCI.4415-14.2015. J Neurosci. 2015. PMID: 26203148 Free PMC article. - Differential corticostriatal plasticity during fast and slow motor skill learning in mice.
Costa RM, Cohen D, Nicolelis MA. Costa RM, et al. Curr Biol. 2004 Jul 13;14(13):1124-34. doi: 10.1016/j.cub.2004.06.053. Curr Biol. 2004. PMID: 15242609 - Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum.
Lovinger DM. Lovinger DM. Neuropharmacology. 2010 Jun;58(7):951-61. doi: 10.1016/j.neuropharm.2010.01.008. Epub 2010 Jan 21. Neuropharmacology. 2010. PMID: 20096294 Free PMC article. Review. - Circuit changes in motor cortex during motor skill learning.
Papale AE, Hooks BM. Papale AE, et al. Neuroscience. 2018 Jan 1;368:283-297. doi: 10.1016/j.neuroscience.2017.09.010. Epub 2017 Sep 14. Neuroscience. 2018. PMID: 28918262 Free PMC article. Review.
Cited by
- Dynamic modulation of spike timing-dependent calcium influx during corticostriatal upstates.
Evans RC, Maniar YM, Blackwell KT. Evans RC, et al. J Neurophysiol. 2013 Oct;110(7):1631-45. doi: 10.1152/jn.00232.2013. Epub 2013 Jul 10. J Neurophysiol. 2013. PMID: 23843436 Free PMC article. - Cytoskeletal determinants of stimulus-response habits.
Gourley SL, Olevska A, Gordon J, Taylor JR. Gourley SL, et al. J Neurosci. 2013 Jul 17;33(29):11811-6. doi: 10.1523/JNEUROSCI.1034-13.2013. J Neurosci. 2013. PMID: 23864670 Free PMC article. - Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease.
Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW. Petzinger GM, et al. Lancet Neurol. 2013 Jul;12(7):716-26. doi: 10.1016/S1474-4422(13)70123-6. Lancet Neurol. 2013. PMID: 23769598 Free PMC article. - Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism.
Wang X, Bey AL, Katz BM, Badea A, Kim N, David LK, Duffney LJ, Kumar S, Mague SD, Hulbert SW, Dutta N, Hayrapetyan V, Yu C, Gaidis E, Zhao S, Ding JD, Xu Q, Chung L, Rodriguiz RM, Wang F, Weinberg RJ, Wetsel WC, Dzirasa K, Yin H, Jiang YH. Wang X, et al. Nat Commun. 2016 May 10;7:11459. doi: 10.1038/ncomms11459. Nat Commun. 2016. PMID: 27161151 Free PMC article. - Endocannabinoids mediate bidirectional striatal spike-timing-dependent plasticity.
Cui Y, Paillé V, Xu H, Genet S, Delord B, Fino E, Berry H, Venance L. Cui Y, et al. J Physiol. 2015 Jul 1;593(13):2833-49. doi: 10.1113/JP270324. Epub 2015 May 13. J Physiol. 2015. PMID: 25873197 Free PMC article.
References
- Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp. Brain Res. 2002;146:122–126. - PubMed
- Miyachi S, Hikosaka O, Miyashita K, Karadi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp. Brain Res. 1997;115:1–5. - PubMed
- Shiffrin RM, Schneider W. Controlled and automatic human information processing. II. Perceptual learning, automatic attending, and a general theory. Psychol. Rev. 1977;84:127–190.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources