Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities - PubMed (original) (raw)
. 2009 Feb;84(2):197-209.
doi: 10.1016/j.ajhg.2009.01.011. Epub 2009 Feb 5.
Leila Romio, Rahul Chodhari, Robert A Hirst, Sandra C P de Castro, Keith A Parker, Patricia Ybot-Gonzalez, Richard D Emes, Stephen W Wilson, Colin Wallis, Colin A Johnson, Rene J Herrera, Andrew Rutman, Mellisa Dixon, Amelia Shoemark, Andrew Bush, Claire Hogg, R Mark Gardiner, Orit Reish, Nicholas D E Greene, Christopher O'Callaghan, Saul Purton, Eddie M K Chung, Hannah M Mitchison
Affiliations
- PMID: 19200523
- PMCID: PMC2668031
- DOI: 10.1016/j.ajhg.2009.01.011
Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities
Victoria H Castleman et al. Am J Hum Genet. 2009 Feb.
Abstract
Primary ciliary dyskinesia (PCD) is a genetically heterogeneous inherited disorder arising from dysmotility of motile cilia and sperm. This is associated with a variety of ultrastructural defects of the cilia and sperm axoneme that affect movement, leading to clinical consequences on respiratory-tract mucociliary clearance and lung function, fertility, and left-right body-axis determination. We performed whole-genome SNP-based linkage analysis in seven consanguineous families with PCD and central-microtubular-pair abnormalities. This identified two loci, in two families with intermittent absence of the central-pair structure (chromosome 6p21.1, Zmax 6.7) and in five families with complete absence of the central pair (chromosome 6q22.1, Zmax 7.0). Mutations were subsequently identified in two positional candidate genes, RSPH9 on chromosome 6p21.1 and RSPH4A on chromosome 6q22.1. Haplotype analysis identified a common ancestral founder effect RSPH4A mutation present in UK-Pakistani pedigrees. Both RSPH9 and RSPH4A encode protein components of the axonemal radial spoke head. In situ hybridization of murine Rsph9 shows gene expression restricted to regions containing motile cilia. Investigation of the effect of knockdown or mutations of RSPH9 orthologs in zebrafish and Chlamydomonas indicate that radial spoke head proteins are important in maintaining normal movement in motile, "9+2"-structure cilia and flagella. This effect is rescued by reintroduction of gene expression for restoration of a normal beat pattern in zebrafish. Disturbance in function of these genes was not associated with defects in left-right axis determination in humans or zebrafish.
Figures
Figure 1
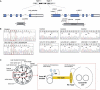
Central-Pair Agenesis in PCD Patients and Linkage Analysis (A) PCD central-pair-defect pedigrees. Black indicates affected; double line indicates consanguineous union, with a dashed upper line if the exact degree of relatedness is unknown; arrow indicates proband. (B) Transmission electron micrographs of nasal ciliary epithelium from individuals with a central-pair defect. Left panel, Bedouin UCL146 IV:1 longitudinal section with intermittent central-pair loss, confirming the previous report of 9+2 (normal) and 9+0 cross-sections. Right panels, cross-sections from UK-Pakistani UCL170 IV:1, indicative of complete central-pair loss (transposition defect), showing 9+2 (upper panel), 9+0 (middle panel), or 8+1 (lower panel) ultrastructure. (C) Linkage mapping of families with central-pair defects, to two loci, on chromosome 6p21.1 and 6q22.1. Upper panel, multipoint MERLIN linkage analysis of UCL146 and UCL152 on 6p21.1 across D6S291_–_D6S1638, with the use of information from Illumina and deCODE scans and in-house microsatellite genotyping. The overlapping shared region of IBD from D6S400 to rs3734693 is reflected by a significant multipoint LOD score > 3, which rises to a peak of 6.7 across D6S1604–D6S451. The location of RSPH9 is shown. Lower panel, multipoint GENEHUNTER linkage analysis of families with transposition defect on 6q22.1 across chromosome 6, with the use of Illumina scan information. The location of RSPH4A is shown, located centromeric to the peak homogeneity LOD score of 7.0, which was generated across markers rs873460–rs941815. (D) Identical-by-descent homozygosity and founder effect in RSPH4A families. Chromosome 6q22.1 disease-chromosome haplotypes for each of the four UK-Pakistani families are shown, displaying extended homozygosity across the RSPH4A locus. Bold, black lines and boxes indicate the linked region in each pedigree, defined by recombination events or loss of homozygosity. The minimal critical region containing RSPH4A is rs2030926_–_rs937091 (defined by recombinations in UCL138). Homozygous allele sharing occurs among affected individuals (gray shading), with a putative minimal common ancestral haplotype spanning the RSPH4A gene, across markers rs1158747–rs2243379 (light gray).
Figure 2
RSPH9 and RSPH4A Mutations (A) Chromosome 6 location of RSPH9 (left) and RSPH4A (right), their genomic structure showing the 5′ and 3′ UTRs (white), introns and exons (blue), their derived proteins (grey), and mutations. (B) Electropherograms indicate the normal sequence (top traces) and mutations (bottom traces). (C) “9+2” cilia axoneme model (cross-section) with putative RSPH9 and RSPH4A location, based on Chlamydomonas homology. Abbreviations are as follows: CP, central microtubular pair; RS, radial spoke; IDA, inner dynein arm; MT, microtubule.
Figure 3
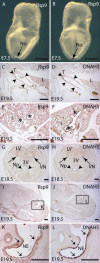
Expression of Rsph9 in the Node and Ciliated Epithelia Whole-mount in situ hybridization of Rsph9 reveals specific expression in the node (No) of E7.5 mouse embryos (arrow); side (A) and anterior (B) views. Expression of Rsph9 (C, E, G, I, K) and Dnah5 (D, F, H, J, L) detected by in situ hybridization on sagittal (C–F and I–L) and coronal (G and H) sections at E18.5 (G and H) and E19.5 (C–F and I–L). Similar expression of both genes was detected in the epithelia lining the trachea (T) (arrowheads in C and D), the bronchi (asterisks in E and F), and the nasopharynx (Np) and neuroepithelium of the lateral ventricles (VN) (G and H). Expression was also prominent in the olfactory epithelium (I and J, magnified in K and L). Abbreviations are as follows: L, lung; LV, lateral ventricle; NE, nasal epithelium; 3V, third ventricle. Scale bar represents 500 μm.
Figure 4
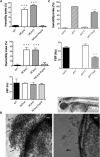
Loss of Zebrafish and Chlamydomonas RSPH9 Gene Function, Causing Dysmotility of Cilia and Flagella (A) Olfactory-pit cilia movement in WT zebrafish and rsph9 morphants. Top panel: 18.2% (Moex2), 22.2% (MOex3), and 0.8% (MOex2 + rsph9 mRNA) of cilia in morphants were immotile (1.4% in WT). Middle panel: 65.9% (MOex2), 65.6% (MOex3), and 7% (MOex2 + rsph9 mRNA) of cilia were dysmotile (3.1% in WT), this number including that which was immotile in addition to dysmotile; i.e., displaying an ineffective circular beat pattern. Bottom panel: cilia beat frequency was unaffected at 42.3 Hz (MOex2), 40.0 Hz (MOex3), and 39.8 Hz (MOex2 + rsph9 mRNA) (WT 43.6 Hz). Means ± SEM from seven WT, eight MOex2, nine MOex3 and five MOex2 plus coinjected rsph9 mRNA embryos are shown. Triple asterisk indicates p < 0.001. (B) Accumulation of debris in rsph9 zebrafish morphant olfactory pit. Top panel, 72 hpf zebrafish, indicating nasal pit in relation to the eye (arrowhead). Bottom left panel: representative example of 72 hpf rsph9 MOex3 morphant zebrafish with debris accumulation evident in the nasal pit (arrows). Bottom right panel: WT zebrafish showing normal debris clearance due to fluid vortex created by cilia beating. MOex2 showed the same defect (not shown). (C) Chlamydomonas flagella movement in WT (cw15) and in pf17, _pf17_-T and _pf17_-Tmut strains. Top: flagella motility was within the normal range at 4.6% in _pf17_-T, but in _pf17_-Tmut, 73.0% of flagella were immotile, compared to 1.0% in WT and 99.8% in pf17. Bottom: flagella beat frequency was within the normal range at 53.5 Hz in _pf17_-T, but reduced to 24.9 Hz in _pf17_-Tmut (WT 47.9 Hz, pf17 0.0 Hz). Shown are means ± SEM from 5–10 cells (immotility index) or 20–26 cells (flagellar beat frequency [FBF]). Triple asterisk indicates p < 0.001.
Similar articles
- Mutations in radial spoke head genes and ultrastructural cilia defects in East-European cohort of primary ciliary dyskinesia patients.
Ziętkiewicz E, Bukowy-Bieryłło Z, Voelkel K, Klimek B, Dmeńska H, Pogorzelski A, Sulikowska-Rowińska A, Rutkiewicz E, Witt M. Ziętkiewicz E, et al. PLoS One. 2012;7(3):e33667. doi: 10.1371/journal.pone.0033667. Epub 2012 Mar 20. PLoS One. 2012. PMID: 22448264 Free PMC article. - Immunofluorescence Analysis and Diagnosis of Primary Ciliary Dyskinesia with Radial Spoke Defects.
Frommer A, Hjeij R, Loges NT, Edelbusch C, Jahnke C, Raidt J, Werner C, Wallmeier J, Große-Onnebrink J, Olbrich H, Cindrić S, Jaspers M, Boon M, Memari Y, Durbin R, Kolb-Kokocinski A, Sauer S, Marthin JK, Nielsen KG, Amirav I, Elias N, Kerem E, Shoseyov D, Haeffner K, Omran H. Frommer A, et al. Am J Respir Cell Mol Biol. 2015 Oct;53(4):563-73. doi: 10.1165/rcmb.2014-0483OC. Am J Respir Cell Mol Biol. 2015. PMID: 25789548 Free PMC article. - Targeted NGS gene panel identifies mutations in RSPH1 causing primary ciliary dyskinesia and a common mechanism for ciliary central pair agenesis due to radial spoke defects.
Onoufriadis A, Shoemark A, Schmidts M, Patel M, Jimenez G, Liu H, Thomas B, Dixon M, Hirst RA, Rutman A, Burgoyne T, Williams C, Scully J, Bolard F, Lafitte JJ, Beales PL, Hogg C, Yang P, Chung EM, Emes RD, O'Callaghan C; UK10K; Bouvagnet P, Mitchison HM. Onoufriadis A, et al. Hum Mol Genet. 2014 Jul 1;23(13):3362-74. doi: 10.1093/hmg/ddu046. Epub 2014 Feb 11. Hum Mol Genet. 2014. PMID: 24518672 Free PMC article. - Genetic causes of bronchiectasis: primary ciliary dyskinesia.
Morillas HN, Zariwala M, Knowles MR. Morillas HN, et al. Respiration. 2007;74(3):252-63. doi: 10.1159/000101783. Respiration. 2007. PMID: 17534128 Review. - Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine: Genetic defects with normal and non-diagnostic ciliary ultrastructure.
Shapiro AJ, Leigh MW. Shapiro AJ, et al. Ultrastruct Pathol. 2017 Nov-Dec;41(6):373-385. doi: 10.1080/01913123.2017.1362088. Epub 2017 Sep 15. Ultrastruct Pathol. 2017. PMID: 28915070 Free PMC article. Review.
Cited by
- The structural heterogeneity of radial spokes in cilia and flagella is conserved.
Lin J, Heuser T, Carbajal-González BI, Song K, Nicastro D. Lin J, et al. Cytoskeleton (Hoboken). 2012 Feb;69(2):88-100. doi: 10.1002/cm.21000. Epub 2012 Jan 12. Cytoskeleton (Hoboken). 2012. PMID: 22170736 Free PMC article. - Splice-site mutations in the axonemal outer dynein arm docking complex gene CCDC114 cause primary ciliary dyskinesia.
Onoufriadis A, Paff T, Antony D, Shoemark A, Micha D, Kuyt B, Schmidts M, Petridi S, Dankert-Roelse JE, Haarman EG, Daniels JM, Emes RD, Wilson R, Hogg C, Scambler PJ, Chung EM; UK10K; Pals G, Mitchison HM. Onoufriadis A, et al. Am J Hum Genet. 2013 Jan 10;92(1):88-98. doi: 10.1016/j.ajhg.2012.11.002. Epub 2012 Dec 20. Am J Hum Genet. 2013. PMID: 23261303 Free PMC article. - Molecular basis of the morphogenesis of sperm head and tail in mice.
Yogo K. Yogo K. Reprod Med Biol. 2022 May 23;21(1):e12466. doi: 10.1002/rmb2.12466. eCollection 2022 Jan-Dec. Reprod Med Biol. 2022. PMID: 35619659 Free PMC article. Review. - Primary ciliary dyskinesia: evaluation using cilia beat frequency assessment via spectral analysis of digital microscopy images.
Olm MA, Kögler JE Jr, Macchione M, Shoemark A, Saldiva PH, Rodrigues JC. Olm MA, et al. J Appl Physiol (1985). 2011 Jul;111(1):295-302. doi: 10.1152/japplphysiol.00629.2010. Epub 2011 May 5. J Appl Physiol (1985). 2011. PMID: 21551013 Free PMC article. - CCDC65 mutation causes primary ciliary dyskinesia with normal ultrastructure and hyperkinetic cilia.
Horani A, Brody SL, Ferkol TW, Shoseyov D, Wasserman MG, Ta-shma A, Wilson KS, Bayly PV, Amirav I, Cohen-Cymberknoh M, Dutcher SK, Elpeleg O, Kerem E. Horani A, et al. PLoS One. 2013 Aug 26;8(8):e72299. doi: 10.1371/journal.pone.0072299. eCollection 2013. PLoS One. 2013. PMID: 23991085 Free PMC article.
References
- Rott H.D. Kartagener's syndrome and the syndrome of immotile cilia. Hum. Genet. 1979;46:249–261. - PubMed
- Jeganathan D., Chodhari R., Meeks M., Faeroe O., Smyth D., Nielsen K., Amirav I., Luder A.S., Bisgaard H., Gardiner R.M. Loci for primary ciliary dyskinesia map to chromosome 16p12.1–12.2 and 15q13.1–15.1 in Faroe Islands and Israeli Druze genetic isolates. J. Med. Genet. 2004;41:233–240. - PMC - PubMed
- O'Callaghan C. Innate pulmonary immunity, cilia. Pediatr. Pulmonol. 2004;26(Suppl.):72–73. - PubMed
- Kosaki K., Ikeda K., Miyakoshi K., Ueno M., Kosaki R., Takahashi D., Tanaka M., Torikata C., Yoshimura Y., Takahashi T. Absent inner dynein arms in a fetus with familial hydrocephalus-situs abnormality. Am. J. Med. Genet. A. 2004;129A:308–311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases